WNT_Signaling
发布时间:2019-12-11 15:30 来源:SABiosciences
- 通路
- 概述
Review
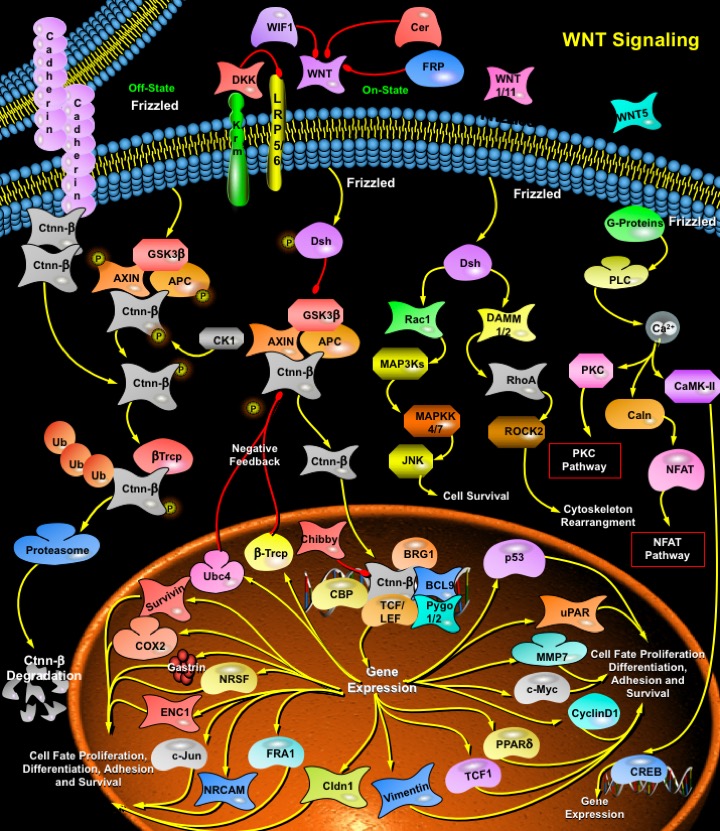
The development of tissues and organs in multicellular organisms is controlled by the interplay of several signaling pathways that cross talk to provide positional information and induce cell fate specification. Together with other families of secreted factors such as TGF-Betas (Transforming Growth Factor-Betas), FGFs (Fibroblast Growth Factors), Hedgehog and Notch proteins, WNT (Wingless-Type MMTV Integration Site Family) Growth Factors are crucially implicated in these processes. The WNT genes encode a large family of secreted protein growth factors that have been identified in animals from Hydra to Human. The name WNT denotes the relationship of this family to the Drosophila segment polarity gene Wg (Wingless) and to its vertebrate ortholog, Int1, a mouse protooncogene. At present, close to 100 WNT genes have been isolated from various species. In humans, 19 WNT proteins have been identified that share 27% to 83% Amino-acid sequence identity and a conserved pattern of 23 or 24 cysteine residues. During development, WNTs have diverse roles in governing cell fate, proliferation, migration, polarity, and death. In adults, WNTs function in homeostasis, and inappropriate activation of the WNT pathway is implicated in a variety of cancers (Ref.1 & 2).
WNT ligands signal via seven transmembrane spanning receptors of the Frizzled family (eleven members in the human genome) together with the recently identified LRP5 (Lipoprotein Receptor-related Protein-5) and LRP6 (Lipoprotein Receptor-related Protein-5) co receptors. Structurally the Frizzled receptors have an Extracellular WNT-binding domain, seven Transmembrane-spanning regions and an Intracellular C-terminal tail. Secondary structure predictions on the Frizzled sequence and involvement of proteins of the WNT signaling pathway with proteins that are characteristic for the pathways of GPCRs (G-Protein Coupled Receptors), labelled Frizzled as a GPCR family member. WNT signals are transduced through at least three distinct intracellular pathways, including the canonical WNT/ Ctnn-Beta (Catenin-Beta) signaling pathway and the 'non-canonical' WNT/Ca2+ (Calcium) pathway, and the WNT/PCP (Planar Cell Polarity) pathway. The WNT/Ctnn-Beta pathway primarily regulates Cell fate determination during development, whereas the major function of the WNT/Polarity pathway is regulation of Cytoskeletal organization. The biological function of the WNT/Ca2+ pathway is unclear (Ref.3).
The WNT/ Ctnn-Beta pathway is the best understood WNT signaling pathway, and is highly conserved during evolution. The WNT/ Ctnn-Beta pathway is activated by WNT1, WNT3, WNT3a, WNT7a, and WNT8, and is involved in transformation. In the absence of WNT signaling, Beta-Ctnn is associated with a cytoplasmic complex containing CK1Alpha (Casein Kinase-1- Alpha), GSK3Beta (Glycogen Synthase Kinase-3-Beta), AXIN (Axis Inhibitor) and the APC (Adenomatous Polyposis Coli) protein. This promotes phosphorylation of Beta-Ctnn and its interaction with Beta-TRCP (Beta-Transducin Repeat-Containing Protein), leading to the ubiquitination of Beta-Ctnn and its degradation by the proteosome (Ref.1). In the presence of WNT signaling, WNT binds to its receptor, Frizzled which leads to activation of the Dsh (Dishevelled) protein. In mammals, the Dishevelled protein family members contains Dsh1, Dsh2, Dsh3 in all organs. These family members have three highly conserved domains that include an N-terminal DIX domain named for Dsh and AXIN, a central PDZ domain termed for Postsynaptic Density-95, Discs-large and Zonula occludens-1, and a C-terminal DEP. The activated Dishevelled protein enhances the phosphorylation of GSK3Beta, which inhibits the ability of GSK3Beta leading to accumulation of free and unphosphorylated Beta-Ctnn in the cytoplasm, which then translocate to the nucleus. In the nucleus prior to WNT signaling, LEF (Lymphoid-Enhancing Factor) and TCF (T-Cell Factor) homolog bind to DNA with sequence specificity in promoter/enhancer regions of target genes, and along with Groucho and CTBP (COOH-terminal Binding Protein), often function to repress gene expression. Elevation of Beta-Ctnn levels by WNT signaling leads to binding of Beta-Ctnn to TCF/LEF, promoting changes in the transcriptional machinery that lead to activation of several target genes. The shifting of proteins from the Cadherin-bound pool to the cytoplasmic pool can increase the amount of available free Ctnn-Beta for the activation of target genes. Transcriptional activation is mediated by the interaction of Ctnn-Beta with the histone acetyl transferase CBP (CREB-Binding Protein), the chromatin-remodeling SWI/SNF complex and Bcl9 bound to Pyg (Pygopus) and BRG1. Chibby interacts directly with the C-terminal region of Ctnn-Beta and inhibits Ctnn-Beta-mediated transcriptional activation by competing with LEF1 to bind to Ctnn-Beta (Ref.4, 5 & 6).
Several genes have now been identified as the target of Ctnn-Beta/TCF transcriptional regulation. These include MMP7 (Matrix Metalloproteinase-7), UPAR (Urokinase-type Plasminogen Activator Receptor), CD44, c-Myc, c-Jun, FRA1 (Fos-Related Antigen-1), CcnD1 (Cyclin-D1), PPAR-Delta (Peroxisome Proliferative Activated Receptor-Delta), TCF1 (Transcription Factor-1), Fibronectin , Slug , Gastrin, Cox2 (Cyclooxygenase-2) and the Gamma2 chain of Laminin5. In addition, the complexes of TCF/LEF and Ctnn-Beta may cooperate with factors activated by other signaling pathways to alter cellular remodeling processes. Many of these genes, including CcnD1 and c-Myc have crucial roles in cell growth, proliferation and differentiation, and are inappropriately activated in colon cancer. c-Myc gene induced by Ctnn-Beta may induce expression of p53, and p53 upregulated p21WAF1 and p130/RB2, resulting in growth arrest. Another target of Ctnn-Beta is Vimentin, a protein involved in cell migration. Vimentin is a direct target of the Ctnn-Beta /TCF transactivation pathway in human mammary tumor cells. WNT signaling can prevent apoptosis by up-regulating anti-apoptotic proteins, such as the Caspase inhibitor, Survivin, and stimulate Angiogenesis via up-regulation of VEGF (Vascular Endothelial Growth Factor). Several proteases capable of degrading extracellular matrix, such as Matrilysin/MMP7 and MMP26, as well as cell adhesion molecules such as CD44 and NRCAM (Neuronal Cell Adhesion Molecule) are WNT targets that could aid the tumour cells in invasion and metastasis. Canonical WNT signaling cascade also regulates the NRSF/REST and ENC1 (Ectodermal-Neural Cortex (with BTB-like domain)-1) genes, thereby controlling the progenitor cells. Cldn1 (Claudin-1) is also involved in the Ctnn-Beta -TCF/LEF signaling pathway, and increased expression of Cldn1 may have some role in colorectal tumorigenesis. Several of the WNT targets genes encode components of the WNT signaling transduction system. The increased expression of Beta-TRCP and the Ubiquitin Conjugating enzyme UbC4/5E2, both involved in degradation of Ctnn-Beta, could act as a negative feedback loop in WNT signaling. Recent studies have identified four families of inhibitors of the WNT signaling pathway: FRP (Frizzled-Related Protein), Cer (Cerberus), WIF1 (WNT-Inhibitory Factor-1), and Dkk1 (Dickkopf-1). Cerberus and WIF1 physically interact with and inhibit WNT. FRP inhibits the WNT signaling pathway by physically associating with both WNT and its receptor, Frizzled. WNT signaling is also inhibited by the secreted protein Dkk1, a member of a multigene family. Dkk1 has been demonstrated to inhibit WNT signaling by binding to and antagonizing LRP5/6. There is recent evidence that the transmembrane proteins Krm1 (Kremen-1) and Krm2 (Kremen-2) are high-affinity Dkk1 receptors that functionally cooperate with Dkk1 to block WNT signaling. Krm2 forms a ternary complex with Dkk1 and LRP6, and induces rapid endocytosis and removal of the LRP6 from the plasma membrane (Ref.6, 7 & 8).
The non-canonical WNT signaling pathway, also termed the atypical WNT-Frizzled signaling pathway, has two intracellular signaling cascades that consist of the WNT/ Ca2+ pathway and the WNT/PCP pathway. In the WNT/ Ca2+ pathway, WNT protein consisting primarily of WNT1, WNT5a, and WNT11, binds to Frizzled transmembrane receptors on the cell surface resulting in several cellular processes that involve stimulation of Heterotrimeric G-proteins, which further activates PLC(Phospholipase-C). PLC lead to increased intracellular Ca2+ release, decreased cGMP (cyclic Guanosine Monophosphate) levels, and activation of the two kinases CamKII (Ca2+- Calmodulin-dependent Protein Kinase-II) or Caln (Calcineurin) and PKC (Protein Kinase-C). These processes can stimulate nuclear factor NFAT and other transcription factors like CREB (cAMP Response Element-Binding Protein-1). Thus, the WNT/Ca2+pathway is most likely a G-protein dependent signaling pathway (Ref.3 & 9). In the WNT/PCP pathway, WNT proteins bind to Frizzled transmembrane receptors on the cell surface followed by activating Rho/Rac small GTPase and JNK (Jun N-terminal Kinase) via Dsh to assist in the subsequent regulation of cytoskeletal organization and gene expression. Dsh is connected via Daam1 to downstream effector Rho and ROCK (Rho-Associated Kinase). Rac is directly activated by Dsh, which further activates JNK by activating MAP3Ks (Mitogen-Activated Protein Kinase Kinase Kinase) and MAP2Ks Mitogen-Activated Protein Kinase Kinase) respectively. The product of the WNT target gene Nkd (Naked) was recently identified as an antagonist for WNT signaling that binds to Dsh and blocks Ctnn-Beta but stimulates the JNK pathway (Ref.3, 10 & 11). The WNT-Frizzled signaling pathway plays an important role in the biology of the development of the nervous system. In particular, the WNT-Frizzled signaling pathway is involved in the development of the neural plate with neuronal progenitor cells and with the subsequent anterior-posterior extension of the neural tube. Ultimately, the WNT-Frizzled signaling pathway leads to the development of the brain, spinal cord, and the extension of numerous sub-populations of sensory and motor neurons. Dysfunction of the WNT-Frizzled pathway can lead to neurodegenerative disorders, such as Alzheimer’s disease and heart failure. Through further identification and targeting of the critical elements that shape and control the WNT-Frizzled signaling pathway, a greater understanding of the biological potential of WNT-Frizzled signaling pathway can emerge for the development of new therapeutic options against neurodegenerative and vascular diseases (Ref.12 & 13).
References
- 1
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise.
- 2
- Katoh M, Katoh M. Comparative genomics on Wnt5a and Wnt5b genes.
- 3
- Li F, Chong ZZ, Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system.
- 4
- Urano T. Wnt-beta-catenin signaling in bone metabolism.
- 5
- Souaze F, Viardot-Foucault V, Roullet N, Toy-Miou-Leong M, Gompel A, Bruyneel E, Comperat E, Faux MC, Mareel M, Rostene W, Flejou JF, Gespach C, Forgez P. Neurotensin receptor 1 gene activation by the Tcf/{beta}-catenin pathway is an early event in h
- 6
- Blavier L, Lazaryev A, Dorey F, Shackleford GM, DeClerck YA. Matrix metalloproteinases play an active role in Wnt1-induced mammary tumorigenesis.
- 7
- Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications.
- 8
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling.
- 9
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways.
- 10
- Goldstein B, Takeshita H, Mizumoto K, Sawa H. Wnt signals can function as positional cues in establishing cell polarity.
 关于我们
关于我们
