Transcription_Repression
发布时间:2019-12-11 15:11 来源:SABiosciences
- 通路
- 概述
Review
Transcriptional repression is an essential mechanism in the precise control of gene expression. Transcriptional repressor proteins associate with their target genes either directly through a DNA-binding domain or indirectly by interacting with other DNA-bound proteins. To inhibit transcription in a selective manner, a repressor protein can (1) mask a transcriptional activation domain, (2) block interaction of an activator with other components of the transcription machinery, or (3) displace an activator from the DNA. Furthermore, DNA response elements can exert allosteric effects on transcriptional regulators, such that regulators may activate transcription in the context of one gene, yet repress transcription in another (Ref.1).
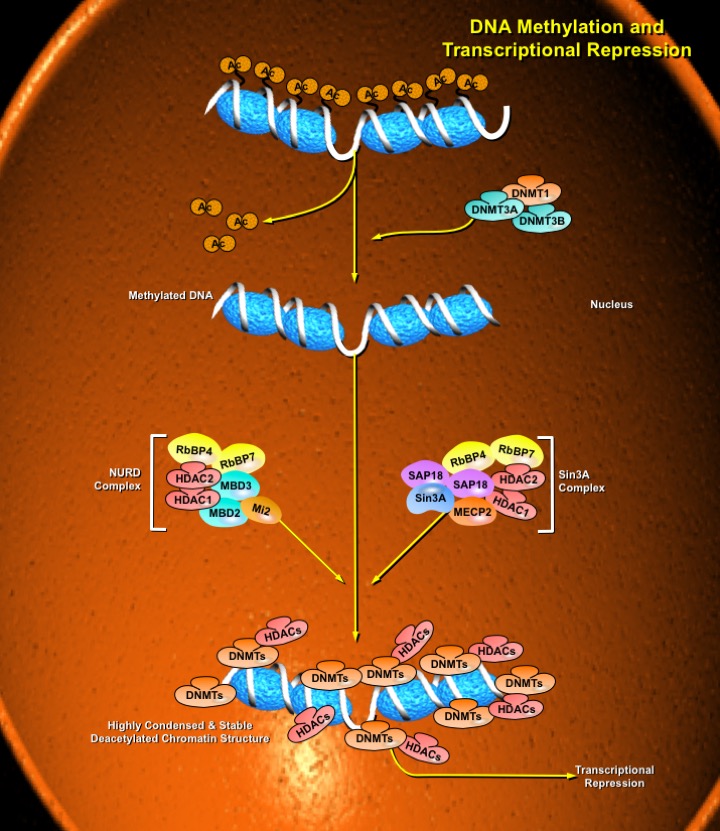
The most direct mechanism by which DNA methylation can interfere with transcription is to prevent the binding of basal transcriptional machinery or ubiquitous TF (Transcription Factors) that require contact with cytosine (C) in the major groove of the double helix. Transcriptionally active chromatin is predominantly unmethylated and has high levels of acetylated histone tails. Most mammalian TFs have GC-rich binding sites and many have CpGs in their DNA recognition elements. Binding by several of these factors is impeded or abolished by methylation of CpG (Ref.2). Methylation at CpG dinucleotides is carried out by one of the three known human DNA methyltransferases (DNMT1, DNMT3a and DNMT3b), resulting in DNA with high levels of CpG methylation, but still containing predominantly acetylated histone tails. CpG methylation induces histone deacetylation, chromatin remodeling and gene silencing through a transcription repressor complex that includes SMRT (Silencing Mediator of Retinoid and Thyroid Receptors), mSin3a, RbAp46/48 and the two histone deacetylases HDAC1 and HDAC2 formed around mSin3a. This complex is assembled by interaction of mSin3a with the methyl-binding protein MeCP2 and SAP18/30 (Sin3-Associated Polypeptides 18/30), which are found associated with large protein complexes such as the NURD complex (Methyl-CpG Binding Domain proteins: MBD2, MBD3). MECP2 acts as a shuttle interlocking DNA methylation and core histone deacetylation in inducing gene silencing. This methyl-binding protein tethers the repression multiprotein complex that includes the corepressor protein, mSin3a, HDAC1 and HDAC2 (Ref.3). The deacetylase activity, which accompanies the MeCP2-bound mSin3a render the promoter of the gene inaccessible to TFs by deacetylating histone H3 and H4. To reverse such a silenced status of a gene, demethylation takes place and an activating complex, which carries the capacity of acetylating histones H3 and H4, replaces the repression complex. This modification of core histones results in a chromatin structure, which is accessible to TFs. Alternatively, the methyl-directed repression can be alleviated by a methylation-overriding effect that is exerted by a strong activation complex ultimately resulting in effective acetylation of histones H3 and H4. In addition to MBD, a TRD (Transcriptional Repressor Domain) overlaps with a region that interacts directly with the corepressor mSin3a (Ref.4). HDAC1 and HDAC2 and chromatin-remodeling activities (Mi-2 and mSin3a) within these complexes result in alterations in chromatin structure, producing chromatin that is refractory to transcriptional activation.
The repression mechanism is significantly different when the number of methylated sites is increased and reaches the threshold that leads to diffusion of gene silencing on the DNA fiber. Hypomethylation contributes to chromosomal instability and possibly to increased expression of some proto-oncogenes. CpG island methylation is capable of silencing tumor suppressor genes and also increases the possibility of mutations, which can occur frequently in these regions. DNA methylation at the 5-position of cytosine within CpG dinucleotides in mammals is essential for important functions, such as cell differentiation, imprinting and X-inactivation. Several genetic diseases are caused by defects within the methylation machinery, like the Rett Syndrome, Fragile X Syndrome and ICF (Immunodeficiency-Centromeric Instability-Facial Anomalies Syndrome).
References
- 1
- Maldonado E, Hampsey M, Reinberg D. Repression: targeting the heart of the matter.
- 2
- Curradi M, Izzo A, Badaracco G, Landsberger N. Molecular mechanisms of gene silencing mediated by DNA methylation.
- 3
- Zlatanova J, Caiafa P, Van Holde K. Linker histone binding and displacement: versatile mechanism for transcriptional regulation.
- 4
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection.
 关于我们
关于我们
