STAT3_Pathway
发布时间:2019-12-11 14:43 来源:SABiosciences
- 通路
- 概述
Review
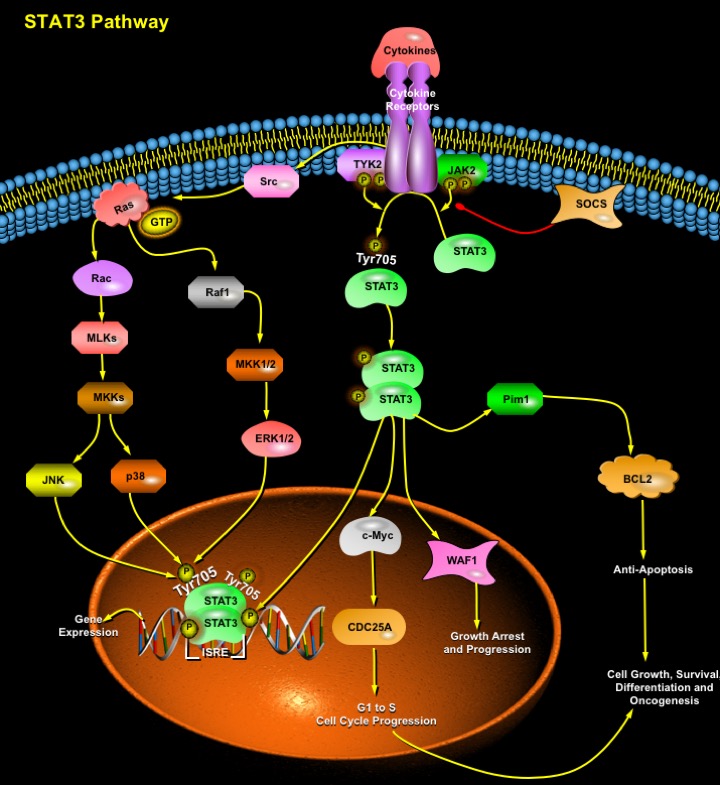
STATs (Signal Transducers and Activators of Transcription) are a family of cytoplasmic proteins with SH2 (Src Homology-2) domains that act as signal messengers and transcription factors and participate in normal cellular responses to Cytokines and GFs (Growth Factors). STATs are activated via the tyrosine phosphorylation cascade after ligand binding and stimulation of the Cytokine Receptor–Kinase complex and Growth Factor-Receptor complex like the EGF (Epidermal Growth Factor), FGF (Fibroblast Growth Factor), PDGF (Platelet-Derived Growth Factor), GCSF (Granulocyte Colony Stimulating Factor), IL-6 (Interleukin-6), CNTF (Ciliary Neurotrophic Factor), OSM (Oncostatin-M), LIF (Leukemia Inhibitory Factor), CSF1R (Colony Stimulating Factor-1 Receptor), c-kit, Insulin receptor, c-Met and GPCRs (G-Protein Coupled Receptors): AgtR2 (Angiotensin-II Receptor). Ligands signaling through the same class of receptor complexes usually activates the same set of STAT factors, e.g. all IL-6-type cytokines activate STAT3 and STAT1 (Ref.1). The activated STATs are subsequently translocated to the nucleus where they bind to specific DNA sites as homo or heterodimers to stimulate transcription of the responsive genes. Among the STAT proteins known to date, STAT3 has been implicated in transduction of the cellular signals that are involved in development of cardiac hypertrophy. The signal-transducing receptor protein GP130 stimulates the JAK (Janus-family tyrosine kinases)-STAT3 pathway and is associated with the regulation of cardiac growth and development (Ref.2).
STAT3 induce gene activation in response to Cytokine Receptor stimulation. Following tyrosine phosphorylation, STAT3 proteins dimerize, translocate to the nucleus, and activate specific target genes like the cis element ISRE (IFN-stimulated Response Element) thereby initiating transcription of several IFN-inducible genes. This transcriptional activation by STAT3 proteins requires the recruitment of coactivators such as CBP (CREB-binding Protein)/p300. STAT3 proteins recognize a conserved element in the promoter of p21/WAF1 (Wildtype p53-Activated Fragment-1) and increase the mRNA expression of this cell cycle regulatory gene. Effectively, STAT3 activates several other genes involved in cell cycle progression such as Fos, Cyclin-D, CDC25A, c-Myc or Pim1 and up-regulates antiapoptotic genes such as BCL2 (B-Cell CLL/Lymphoma-2), BCLXL and Beta2-Macroglobulin (Ref.3). Thus, many STAT3 target genes are key components of the regulation of cell cycle progression from G1 to S phase. Accordingly, STAT3 activation is often associated with cell growth or transformation, and disruption of the STAT3 gene causes embryonic lethality. Following IL-6 stimulation, transcriptional cofactor NCOA/SRC1a interacts with STAT3 and potentiates its transcriptional activity through its CBP/p300-interacting domain AD1 (Ref.4). Pathways other than JAK kinases involving mTOR (mammalian Target of Rapamycin) or p70S6 kinase, MAPK (Mitogen Activated Protein Kinase), p38, and MEK (MAPK/ERK Kinase) signaling cascades also lead to phosphorylation and activation of STAT3 (Ref.5).
RhoA efficiently modulate STAT3 transcriptional activity by inducing its simultaneous tyrosine and serine phosphorylation via Src Family of Kinases and JAK2. The JNK (c-Jun N-terminal Kinase)/ERK (Extracellular-Signal Regulated Kinase) Pathway mediates serine phosphorylation (Ser727) and cooperation of both tyrosine as well as serine phosphorylation is necessary for full activation of STAT3 (Ref.5). The Type-I Interferon (IFN-Alpha/Beta) promotes the DNA-binding activity of the transcription factors including STAT3, which is involved in the induction of NF-KappaB (Nuclear Factor-KappaB) DNA-binding activity and in the induction of antiviral and antiproliferative activity. STAT3 is also activated in response to the small guanine nucleotide-binding protein Rac1. The Rac functions in growth factor-induced activation of STAT3 in two ways. It apparently helps localize STAT3 to kinase complexes at the cell surface through Ras and also promotes activation of kinases, like MLKs (Mixed-Lineage Kinases), JAK2, TYK2 that phosphorylate STAT3 at Tyr705 (Ref.6). The SOCS (Suppressor of Cytokine Signaling) family of proteins negatively regulates the receptor-associated JAK-STAT3 pathway of transcriptional activation.
STATs have been implicated in programming gene expression in biological events such as embryonic development, programmed cell death, organogenesis, innate immunity, adaptive immunity and cell growth regulation in many organisms. Abnormal activity of certain STAT family members, particularly STAT3 is associated with a wide variety of human malignancies, including lymphomas; leukemias; mycoses fungoides and multiple myeloma (Ref.7). Thus, STAT3 activate either of two gene expression programs, one for growth promotion and one for growth arrest. Recently inhibition of the STAT3 signaling pathway using the JAK-selective inhibitor, AG490, and a dominant negative STAT3 (STAT3-Beta) significantly suppressed the growth of ovarian and breast cancer cell lines. These results suggest that inhibition of STAT3 signaling may provide a potential therapeutic approach for treating ovarian and breast cancers (Ref.8).
References
- 1
- Abell K, Watson CJ. The Jak/Stat pathway: a novel way to regulate PI3K activity.
- 2
- Ji JD, Kim HJ, Rho YH, Choi SJ, Lee YH, Cheon HJ, Sohn J, Song GG. Inhibition of IL-10-induced STAT3 activation by 15-deoxy-Delta12,14-prostaglandin J2.
- 3
- Barre B, Avril S, Coqueret O. Opposite Regulation of Myc and p21waf1 Transcription by STAT3 Proteins.
- 4
- Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O. Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a.
- 5
- Aznar S, Valeron PF, del Rincon SV, Perez LF, Perona R, Lacal JC. Simultaneous tyrosine and serine phosphorylation of STAT3 transcription factor is involved in Rho A GTPase oncogenic transformation.
- 6
- Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by direct binding to the Rac1 GTPase.
- 7
- Shen Y, Devgan G, Darnell JE Jr, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1.
- 8
- Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2 pathway mediates interleukin-6-elicited inducible NOS activation and decrease in contractility in adult ventricular myocytes.
 关于我们
关于我们
