Signaling_in_Gap_Junction
发布时间:2019-12-11 12:03 来源:SABiosciences
- 通路
- 概述
Review
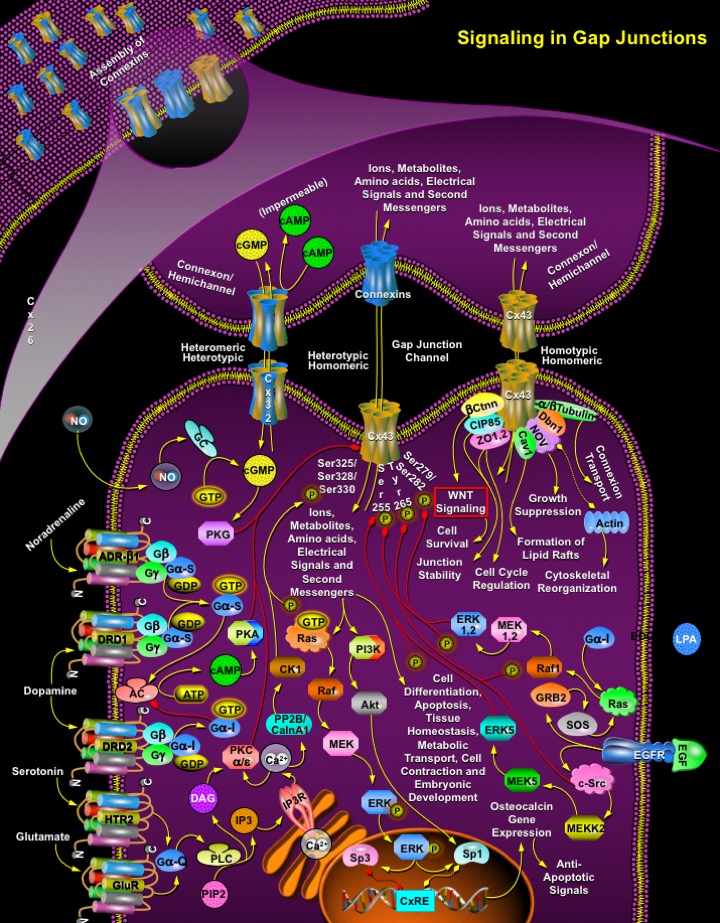
Gap Junction (GJ) channels span two plasma membranes and are formed by the alignment of two hemichannels, each consisting of an oligomer of structural subunit proteins, called Cxs (Connexins). These junctional proteins constitute a multigene family whose members are distinguished according to their predicted molecular weight in kilodaltons. A Connexin structure consists of two extracellular loops (EL), four membrane-spanning domains (TM), one cytoplasmic loop (CL), one N-terminal tail (NT), and one C-terminal tail (CT) (Ref.1 & 2). During intercellular channel formation, six Connexins oligomerize into a Connexon or hemichannel that docks in homotypic, heterotypic and combined heterotypic/heteromeric arrangements. In total, as many as 14 different Connexon arrangements can be formed when two members of the Connexin family intermix. This is followed by Connexon trafficking to the plasma membrane. The intact channel is formed when one hemi-channel docks with a second in an opposing cell. Once assembled, groups of these intercellular channels (termed Gap Junctional plaques) mediate the passage of amino acids, second messengers, electrical signals, ions and other metabolites between the connected cytoplasmic domains (Ref.3).
The function of Gap Junctions in cell and tissue biology is of utmost importance as GJIC (Gap-Junctional Intercellular Communication) exists in nearly every mammalian cell type and among the Connexins, Cx43 (Connexin-43) is the most ubiquitously expressed. Cx43 is endogenously expressed in at least 35 distinct tissues encompassing over 35 cell types that include cardiomyocytes, keratinocytes, astrocytes, endothelial cells and smooth-muscle cells among many others (Ref.4 & 5). Cx43 co-oligomerize with mostly with other Connexins like Cx26 (keratinocytes and hepatocytes), Cx31 (keratinocytes), Cx45 (myocardium) and Cx46 (trans-Golgi network). However Cx43 is unable to co-oligomerize with Cx32. Besides Connexins, Cx43 binds with ZO-1 (Zona Occludens-1) and ZO-2, at different stages of the cell cycle to regulate Gap Junctions size and stability; interacts directly with Ctnn-Beta (Catenin-Beta) to regulate GJIC cross-talk activity with the WNT signaling (essential for cell survival); further binding of CIP85 (Cx43-Interacting Protein of 85 kDa) to two proline-rich motifs of Cx43 and regulates the turnover of Cx43-containing Gap Junctions, stabilizing the junctions. The Actin-binding protein Dbn1 (Drebrin-1) binds Cx43 and links Gap junctions to the sub-membrane cytoskeleton, whereas, other cytoskeletal proteins like Tubulin-Alpha and Beta binds with Cx43 to facilitate Connexion transport. Likewise, another potential signaling molecule that bind to the C-terminus of Cx43 is NOV (Nephroblastoma Overexpressed Gene)/CCN3 (CTGF (Connective-Tissue Growth Factor), Cyr61/Cef10 and Nephroblastoma Overexpressed Gene), regulating growth suppression. Similarly, Cx43 binds to Cav1 (Caveolin-1) may play a role in internalization of Cx43. Apart from Cx43 functions, the first example of Connexin-specific selectivity among second messengers is observed when homomeric Connexons made up of Cx32 are permeable both to cAMP (Cyclic Adenosine 3',5'-Monophosphate) and cGMP (Cyclic Guanosine Monophosphate) but heteromeric Connexons containing both Cx26 and Cx32 are not permeable to cAMP but allow passage of cGMP (Ref.1 & 5).
Consisting of hundreds of intercellular channels, Gap Junctions are critically important in regulating embryonic development, excitable cell contraction, tissue homeostasis, apoptosis, metabolic transport and normal cell growth and differentiation. The Gap Junctional communication is controlled by neurotransmitters (like Noradrenaline, Dopamine, Serotonin and Glutamate), cytokines, growth factors, and other bioactive compounds (like LPA (Lysophosphatidic Acid)). These biomolecules activate the downstream kinases, the kinase activators then significantly increase levels of Cx43 phosphorylation at specific sites leading to channel opening and closure. Kinases like PKG (cGMP-Dependent Protein Kinase), PKA (cAMP-Dependent Protein Kinase), PKC (Protein Kinase-C), CK1 (Casein Kinase-1), ERK1 (Extracellular Signal Regulated Kinase-1), ERK2, ERK5 and c-Src have implicated roles in Cx43 phosphorylation and acute gating of Gap Junction channels (Ref.6 & 7). The neurotransmitter, Dopamine, release modulates cAMP production and PKA activation by activating DRDs (Dopamine Receptors). These receptors work both synergistically and antagonistically to modulate synthesis of cAMP, which results in stimulation and increase of the intracellular concentration of Ca2+ (Calcium Ions), leading to activation of various signaling pathways. When Dopamine/DRD1 and Noradrenaline/ADR-Beta1 (Adrenergic Receptor-Beta-1) gets coupled to GN-AlphaS (GN-AlphaS Complex Locus), GN-Beta (Guanine Nucleotide-Binding Protein-Beta) and GN-Gamma (Guanine Nucleotide-Binding Protein-Gamma) it leads to activation of AC (Adenylyl Cyclase), and cAMP production. In contrast, when Dopamine/DRD2 binds to the GN-AlphaI (Guanine Nucleotide Binding Protein-Alpha Inhibiting Activity Polypeptide)/GN-Beta/GN-Gamma, AC activity is blocked and cAMP production is reduced (Ref.5).
Gating is also controlled by a short-lived free radical gas NO (Nitric Oxide) through GC (Guanylyl Cyclase)-cGMP route activating PKG. Other neurotransmitters like Serotonin and Glutamate bind to HTR2 (5-Hydroxytryptamine-2 Receptor) and GluR (Glutamate Receptor) (both being coupled to GN-AlphaQ (Guanine Nucleotide-Binding Protein-Alpha-Q)) to activate PLC (Phospholipase-C) generating IP3 (Inositol 1,4,5-trisphosphate) and DAG (Diacylglycerol) through PIP2 (Phosphatidylinositol 4,5-bisphosphate) cleavage. DAG activates PKC, whereas binding of IP3 to IP3R (IP3 Receptor) facilitates release of Ca2+ from endoplasmic reticulum. This Ca2+ influx culminates in the stimulation of PKC (Alpha/Epsilon), PP2B (Protein Phosphatase-2B)/CalnA1 (Calcineurin-A1) and CK1 (Casein Kinase-1) activation by PP2B/CalnA1. Activated PKA, PKG and PKC (Alpha/Epsilon) formulate inhibition of Cx43 but CK1 phosphorylates Cx43 on Ser325 (Serine-325), Ser328, or Ser330 residues to activate gating during Gap Junction signaling (Ref.7). Gap junctional communication thus permits the intercellular propagation of ions, amino acids, electrical signals, metabolites and second messengers that activate the ERK/PI3K (Phosphatidylinositde-3-Kinase) signal cascades. On traversing the Gap junctional pore, second messenger activates Ras>Raf (v-Raf Murine Leukemia Viral Oncogene Homolog)>MEK (MAPK/ERK Kinase)>ERK and PI3K>Akt (v-Akt Murine Thymoma Viral Oncogene Homolog) signaling. The activated ERK is subsequently translocated to the nucleus where it phosphorylates Sp1 (Transcription Factor-Sp1) resulting in preferential recruitment to the CxRE and thereby leading to robust gene transcription and a potentiation of the primary response in osteoblastic cells (Ref.8). Further Akt phosphorylation by PI3K may act in a parallel or linear manner with respect to ERK signaling stimulating Sp1 phosphorylation and activation of Osteocalcin gene expression. However disruption of Gap Junctions or decrease in permeability of Gap Junctions (by formation of heteromeric channels (e.g., Cx26/Cx32, which are not permeable to cAMP but allow passage of cGMP or by pharmacological inhibition) leads to loss of Sp1 phosphorylation and results in the preferential recruitment of Sp3, thus ERK cascade dependent phosphorylation of Sp1 also mediates the preferential recruitment of Sp1 over Sp3, where Sp3 acts as a repressor of Sp1-mediated transcriptional activation by deregulating the relay of anti-apoptotic signals to osteoblasts (Ref.9).
Gap junctional communication also gets disrupted in response to extracellular cue like LPA or growth factors that regulates post-translational phosphorylation of Cx43. Phosphorylation and inhibition of Cx43 by various protein kinases that regulates the assembly of Gap Junctions to form functional Connexons in the plasma membrane, Connexin redirection from the plasma membrane, and also alters channel open probability. Growth factors like EGF (Epidermal Growth Factor) coupled to its receptor EGFR (Epidermal Growth Factor Receptor) stimulates the rapid and transient disruption of GJIC, and a marked increase in the phosphorylation of Cx43, on Ser255, Ser279 and Ser282. EGF activation of ERK1/2 through GRB2 (Growth Factor Receptor-Bound Protein-2)>SOS (Son of Sevenless)>Ras>Raf1>MEK1/2 route mediates such Cx43 phosphorylation on Serine residues and subsequent inhibition of GJIC (Ref.7). In a similar manner LPA induced activation of EDG2 (Endothelial Differentiation Lysophosphatidic Acid G-Protein Coupled Receptor-2)/GN-AlphaI (Guanine Nucleotide Binding Protein-Alpha Inhibiting Activity Polypeptide) acts synergistically with EGF/EGFR mediated signaling to facilitate phosphorylation of Serine residues on Cx43 through GN-AlphaI> Ras> Raf1> MEK1/2 activation. However EGF activation of ERK5 is more important than ERK1/2 as it regulates Cx43 Gap Junction uncoupling by association and Cx43 Ser255 phosphorylation via the c-Src>MEKK2 (MAP/ERK Kinase Kinase-2)>MEK5 route, which is sufficient to inhibit rapid gating during GJIC. Here apart from ERK5, c-Src also interact and phosphorylates Cx43 on Tyr265 (Tyrosine-265) (Ref.5).
Controlled gating through Gap Junctions is highly essential for normal functioning of a cell. Mutations (dominant) in the gene encoding Cx43 leads to pleiotropic developmental disorder ODDD (Oculodentodigital Dysplasia). This disorder is related to diseases that range from Syndactyly, craniofacial abnormalities, brittle nails, hair abnormalities, conductive hearing loss, lens defects, cornea defects, abnormalities of the teeth and occasional neurological and heart symptoms. At present, no fewer than eight distinct human diseases have been definitively linked to germline mutations in Connexin family members (Ref.10). Cx43 is the most universal Connexin occurring in the human body and to date, the vast majority of Cx43-binding proteins have been assigned putative functions in regulating some part of the life cycle of Connexins but attention needs to be given to the possibility that whether one or more of these interactions could regulate the gating properties of these channels (Ref.1).
References
- 1
- Laird DW. Life cycle of connexins in health and disease.
- 2
- Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death.
- 3
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions.
- 4
- Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression.
- 5
- Nicholson BJ. Gap junctions - from cell to molecule.
- 6
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly.
- 7
- Cameron SJ, Malik S, Akaike M, Lerner-Marmarosh N, Yan C, Lee JD, Abe J, Yang J. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation.
- 8
- Stains JP, Civitelli R. Gap junctions in skeletal development and function.
- 9
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters.
- 10
- Kelsell DP, Dunlop J, Hodgins MB. Human diseases: clues to cracking the connexin code?
 关于我们
关于我们
