Rho_family_GTPase
发布时间:2019-12-11 11:47 来源:SABiosciences
- 通路
- 概述
Review
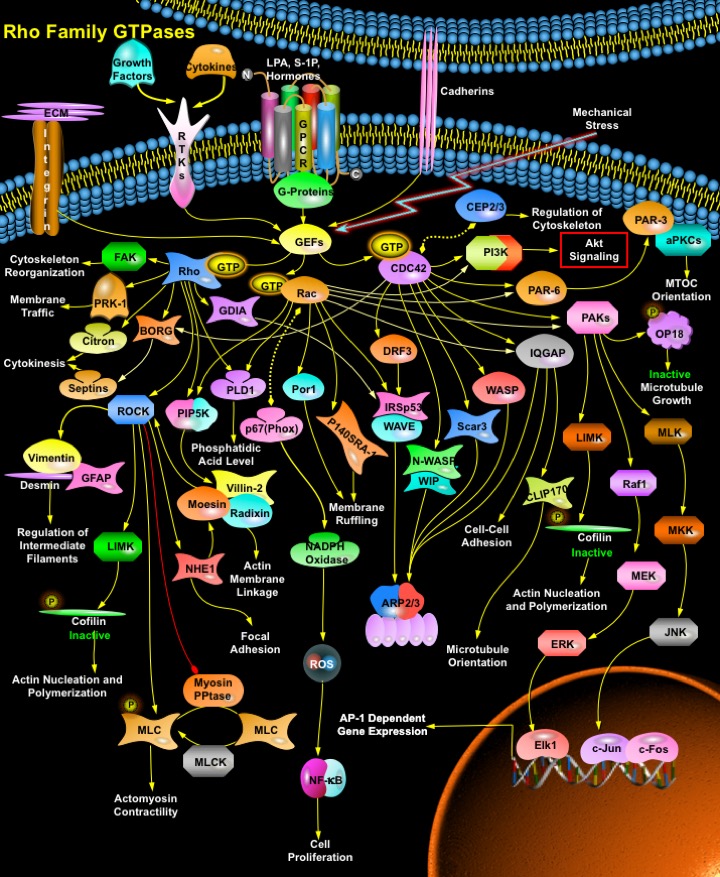
The Rho family of small GTP-binding proteins comprises a group of signaling molecules that are activated by a variety of Growth factors, Cytokines, Adhesion molecules, Hormones, Integrins, G-proteins and other biologically active substances and regulate a wide range of biological processes, including Reorganization of the Actin Cytoskeleton, Transcriptional Regulation, Vesicle Trafficking, Morphogenesis, Neutrophil activation, Phagocytosis and activation of the NADPH Oxidase, Mitogenesis, Apoptosis and Tumorigenesis. The mammalian Rho GTPase family currently consists of three subfamilies, Rho (RhoA, RhoB and RhoC), Rac (Rac1, Rac2 and Rac3) and CDC42 (Cell Division Cycle-42) (CDC42Hs and G25K). The best-characterized family members of Rho Family GTPase are RhoA, Rac1 and CDC42. Each controls the formation of a distinct cytoskeletal element in mammalian cells. Activation of Rac induces Actin polymerization to form Lamellipodia (broad web-like extensions), whereas activation of CDC42 stimulates the polymerization of actin to filopodia or microspikes (long and thin extensions). In contrast, Rho regulates bundling of actin filaments into stress fibers and the formation of focal adhesion complexes. The small GTPases of the Rho family act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state, a process that is regulated by GEFs (Guanine nucleotide Exchange Factors) and GAPs (GTPase Activating Proteins). GEFs catalyze the conversion to the GTP-bound state and GAPs accelerate the intrinsic rate of hydrolysis of bound GTP to GDP. Additionally, GDIs (GDP-Dissociation Inhibitors) have been described that capture Rho in both GTP and GDP-bound states and allow it to cycle between cytosol and membranes. In its active state Rho GTPases interact with their specific downstream targets and perform their cellular functions (Ref.1, 2 & 3).
The major activator of Rho family GTPase is GPCR (G-Protein coupled Receptor). GPCRs that activate Rho and use G-Alpha12 or G-Alpha13 for signal transduction include receptors for LPA (Lysophosphatidic Acid) and S-1P (Sphingosine 1-Phosphate) and certain Hormones. Besides GPCR, Itgs (Integrins) also activate Rho GTPase. Integrins both directly activate and enhance growth factor activation of Rac and CDC42 through an effect on membrane targeting. Integrins contribute to activation of PI3K (Phosphatidylinositde-3 Kinase), possibly via FAK (Focal Adhesion Kinase) and CDC42, and promote polymerization and organization of actin filaments through both direct physical connections and a variety of signaling pathways. Growth factors and Cytokines also activate Rho GTPases through interactions with GAPs, GDIs, GDFs (GDI-Dissociation Factors) and GEFs. Activation of the GEFs by tyrosine Kinase growth factor receptors leads to exchange of GDP for GTP, thus activating Rho. Besides, certain mechanical stresses and Cadherin group of transmembrane glycoproteins also activates Rho GTPases via activating GEFs. Activated CDC42, Rac and Rho finally bind to and specifically activate their downstream effectors (Ref.4 & 5).
A number of proteins have been identified as targets of Rho. These targets include the PAK (p21-Activated Kinase) family, Rho-kinase/ROK/ROCK (Rho-Associated Coiled-Coil-Containing Protein Kinase), MBS (Myosin-Binding Subunit) of Myosin PPtase (Myosin Phosphatase), PKN (Protein Kinase-N)/PRK-1, Rhophilin, Rhotekin, Citron, and GDIA. ROCK is the major target of Rho. ROCK phosphorylates both MLC (Myosin Light Chain) and MBS, which lead to inactivation of Myosin PPtase, thus playing an important role in Actomyosin Contractility. ROCK also activates LIMK (LIM-Kinase). Both LIMK1 and 2 phosphorylate and inactivate Cfl (Cofilin), an Actin-depolymerizing factor, and regulates Actin Cytoskeletal reorganization. ROCK, can also phosphorylate ERM (Ezrin/ Vil (Villin), Rdx (Radixin) and Msn (Moesin)) proteins in vitro but the effect of ROCK inhibitors on ERM phosphorylation in vivo varies and it is unclear whether ROCK acts directly or indirectly to modify ERM phosphorylation. ROCK can also phosphorylate the sodium–hydrogen exchanger, NHE1 (Na-H Exchanger-1) (Ref.1, 6 & 7). NHE1, on the other hand, interacts with ERM proteins both directly and via EBP50 (Ezrin-Radixin-Moesin Binding Phosphoprotein-50). ERM Proteins can also be activated by Rho via PIP5K (Phosphatidylinositol-4-Phosphate 5-Kinase). Both Rac and Rho bind to and activate PIP5K, which increases the amount of PIP2 (Phosphatidylinositol (4,5)-bisphosphate). PIP2 then activates ERM proteins by inhibiting their interdomain interaction, which allows phosphorylation of their C-terminal threonine residue by some kinases. The C-terminally threonine-phosphorylated ERM proteins are stabilized at the activated forms, which function as Actin filament/plasma membrane cross-linkers to form microvilli. Activated ERM proteins are associated directly with the Adhesion molecules such as CD44 and ICAM-1 (Intercellular Adhesion Molecule-1), -2, and -3 and indirectly with other integral membrane proteins such as NHE3 (Sodium/Hydrogen Exchanger-3) through EBP50/NHERF (Sodium-Hydrogen Exchanger Regulatory Factor). Activated ERM proteins also bind to Rho-GDI at their N-terminal halves, suppressing GDI activity of Rho-GDI to release GDP-Rho, which is activated to GTP-Rho. This GTP-Rho can be used to activate ERM proteins just beneath the plasma membranes, providing a positive feedback pathway. ROCK also phosphorylates Type III intermediate filaments including Vim (Vimentin), GFAP (Glial Fibrillary Acidic Protein) and Des (Desmin). These effects of ROCK have been linked to reorganization of intermediate filaments at cytokinesis. Other reported substrates for Rho kinases include MARCKS, EF-1Alpha, Calponin, and CPI-17 and Collapsin-Response Mediator Protein-2 (Ref.8 & 9).
Besides ROCK, other important targets of Rho include FAK, PRK-1/ PKN1, BORG (Binder of Rho GTPases), Citron, PLD (Phospholipase-D) and GDIA. The GTPase RhoA plays a prominent role in regulating the organization of the cytoskeleton by promoting the assembly of focal adhesions and actin stress fibers and by activating FAK. PKN1/PRK-1 and PKN2 are Rho effectors involved in endosomal trafficking. Citron is a ROCK-related kinase that is critical for cytokinesis and is also implicated in other aspects of cell cycle progression. BORG proteins are Rho effectors that connect to Septs (Septins), structural proteins that can polymerize to form filaments involved in cytokinesis in yeast and mammalian cells, and that probably carry out additional structural roles in mammalian cells as well. Members of the Rho subfamily of GTP-binding proteins including Rho and Rac are implicated in the regulation of PLD. PLD catalyzes the hydrolysis of Phosphatidylcholine to yield Phosphatidic Acid and Choline. Phosphatidic Acid is a second messenger involved in membrane remodeling events that are critical to cell growth, such as vesicle trafficking and regulated secretion. Rho also activates scaffolding proteins such as GDIA, WASP (Wiskott-Aldrich syndrome protein) and IRSp53 (Insulin Receptor Substrate Protein-53) (Ref.10 & 11).
Like Rho, Rac and CDC42 also have numerous effectors that mediate effects on the cytoskeleton and gene expression. Rac binds p67(Phox) to increase activation of the NADPH Oxidase system and production of ROS (Reactive Oxygen Species), which mediate activation of NF-KappaB (Nuclear Factor-KappaB)-dependent gene expression, effects of Rac on cell cycle progression and inhibition of Rho activity. Rac binds the WAVE (WASP Family Verprolin Homology Domain-Containing Protein) ) complex (also containing Abi and IRSp53/58), to release active WAVE, which promotes actin polymerization in lamellipodia through activation of the ARP2/3 (Actin-Related Protein-2/3) complex. Both Rac and CDC42 bind and activate the kinases PAK1, PAK2 and PAK3. PAKs have multiple substrates, including LIMK, which leads to Actin polymerization; OP18 (Oncoprotein-18)/Stathmin, which stabilizes microtubule plus ends; and Raf1 and MEK1 (MAPK/ERK Kinase-1), whose phosphorylation by PAK enhances transmission of the signal to ERK (Extracellular Signal-Regulated Kinase). PAKs have also been reported to activate the JNK (c-Jun Kinase) via MLKs (Mixed Lineage Kinases) and MKKs (Mitogen-Activated Protein Kinase Kinases). Activation of JNK causes phosphorylation and activation of several transcription factors, including c-Jun, c-Fos and Elk1. PAK activity also regulates myosin phosphorylation and cell contractility through several pathways, including myosin light chain kinase, myosin regulatory light chain, myosin heavy chain and Caldesmon. Rac and CDC42 also bind to the actin-binding protein IQGAP, which is implicated in regulation of cell-cell adhesion and Microtubule orientation. By binding to the microtubule tip protein Clip170, IQGAP1 captures growing microtubules at the leading edge of migrating fibroblasts, which results in cell polarization. Recently, a novel Rac-interacting protein, POR1 (Partner of Rac1), has been shown to play a role in membrane ruffling. p140SRA1 (Specifically Rac1-Associated Protein) is also a novel Specific target for Rac1 Small GTPase and is also involved in membrane ruffling. Both Rac and CDC42 also bind and stimulate PI3K, which activates Akt. WASP (Wiskott-Aldrich Syndrome Protein) (and the more widely expressed N-WASP (Neural Wiskott-Aldrich Syndrome Protein) and WIP (WASP-Interacting Protein)) are critical downstream effectors of CDC42 that mediate formation of filopodia. These effectors also require PtdIns (4,5)P2 and interact directly with ARP2/3 complex to promote actin polymerization. Recent findings show that CDC42 and Rac, involved in the dynamics of Actin cytoskeleton and cell polarity, bind to a protein complex containing PAR-6, PAR-3/ASIP, and aPKC (atypical Protein Kinase-C). Other downstream effectors of CDC42 include SCAR3 (Suppressor of cAMP Receptor-3), IRSp53 , IQGAP1, MRCK/ CDC42BPA (CDC42 Binding Protein Kinase Alpha (DMPK-like), POR1, DRF3 (Diaphanous Related Formin-3) and CEP2/3 (CDC42 Effector Protein-2/3), also called the BORGs (Ref. 2, 12 & 13). Signaling pathways that are regulated by Rho family members play an important role in several pathological conditions, including cancer, inflammation, and bacterial infections. CDC42 mediates cell polarity in several systems including migrating cells and early embryos, which involves reorientation of the MTOC (Microtubule Organizing Center) and Golgi apparatus toward the direction of movement. CDC42 is a regulator for multiple aspects of dendritic morphogenesis. The Rho GTPases have provided an important experimental model for GTPase-mediated signalling that highlights the multiple levels of regulation that modulate their activity as signal-transducing molecules. Although substantial evidence indicates that the balance between the two nucleotide-bound states of these proteins correlates well with their ability to promote biological responses, the precise mechanism by which this balance is regulated is still largely unknown. Moreover, although it is clear that a discrete ‘on–off’ switch is too simple a mechanism to account for the current experimental evidence, whether the regulated intracellular translocation of Rho family GTPases has a role still needs to be elucidated. Finally, the precise role of GTP hydrolysis in the normal mode of action of these GTPases also remains to be addressed. The unexpected roles of Rho GTPases in regulating hematopoietic development and maturation point to new areas for future investigation. In addition, how Rho GTPases might modulate the responses of the innate immune system to infectious pathogens and agents of bioterrorism are particularly relevant issues in our current world situation (Ref.14 & 15).
References
- 1
- Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho Family of Ras-like GTPases in Eukaryotes.
- 2
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking.
- 3
- Oleksy A, Opalinski L, Derewenda U, Derewenda ZS, Otlewski J The molecular basis of RhoA specificity in the guanine nucleotide exchange factor PDZ-RhoGEF.
- 4
- Brazier H, Stephens S, Ory S, Fort P, Morrison N, Blangy A. Expression profile of RhoGTPases and RhoGEFs during RANKL-stimulated osteoclastogenesis: identification of essential genes in osteoclasts.
- 5
- Bourdon DM, Wing MR, Edwards EB, Sondek J, Harden TK. Quantification of isozyme-specific activation of phospholipase C-beta2 by Rac GTPases and phospholipase C-epsilon by Rho GTPases in an intact cell assay system.
- 6
- Kang JH, Jiang Y, Toita R, Oishi J, Kawamura K, Han A, Mori T, Niidome T, Ishida M, Tatematsu K, Tanizawa K, Katayama Y. Phosphorylation of Rho-associated kinase (Rho-kinase/ROCK/ROK) substrates by protein kinases A and C.
- 7
- Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis.
- 8
- Torka R, Thuma F, Herzog V, Kirfel G. ROCK signaling mediates the adoption of different modes of migration and invasion in human mammary epithelial tumor cells.
- 9
- Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction.
- 10
- Lim WG, Tan BJ, Zhu Y, Zhou S, Armstrong JS, Li QT, Dong Q, Chan E, Smith D, Verma C, Tan SL, Duan W. The very C-terminus of PRK1/PKN is essential for its activation by RhoA and downstream signaling.
 关于我们
关于我们
