Retinoic_Acid_Mediated_Apoptosis
发布时间:2019-12-11 11:44 来源:SABiosciences
- 通路
- 概述
Review
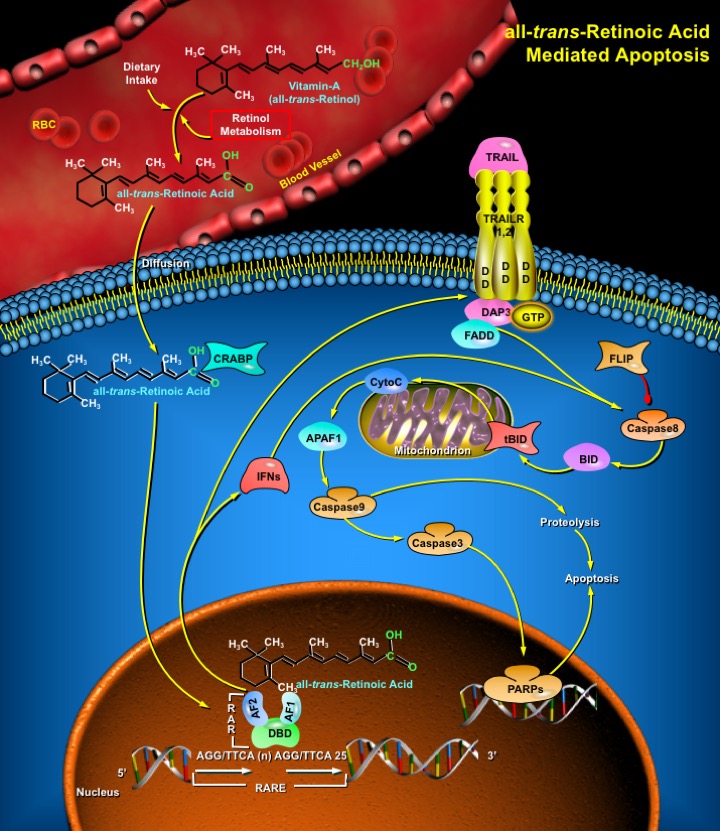
Retinoic Acid, a lipophilic molecule and a metabolite of Vitamin-A (all-trans-Retinol), affects gene transcription and modulates a wide variety of biological processes like Cell Proliferation, Differentiation, including Apoptosis. Retinoic Acid mediated gene transcription depends on the rate of transport of Retinoic Acid to target cells and the timing of exposure of Retinoic Acid to RARs (Retinoic Acid Receptors) in the target tissues. The all-trans-Retinoic Acid, the Carboxylic Acid form of Vitamin-A is of biological significance since it has high circulating levels than other isomers of Retinoic Acid. The targets of all-trans-Retinoic Acid and RARs include a multitude of Structural genes, Oncogenes, Transcription Factors and Cytokines. Although biologically active ligands for the RARs also include 9-cis-Retinoic Acid among others, yet circulating levels of 9-cis-Retinoic Acid are much lower than those of all-trans-Retinoic Acid and the physiological significance of the isomerization all-trans-Retinoic Acid to 9-cis-Retinoic Acid and vice versa is yet to be ascertained (Ref.1). The all-trans-Retinoic acid is predominant under most physiological situations and explains all of the biological effects of Vitamin-A. The RARs are encoded by three separate genes with multiple isoforms-Alpha, Beta and Gamma, which are generated by alternative promoters and differential splicing. Like all NRs (Nuclear Receptors) RARs also have a conserved modular structure consisting of an AF-1 or A/B (Amino-Terminal Activating Factor-1 Transcriptional Activation) Domain; a zinc-finger DBD or C (DNA-Binding Domain); a CoR or D (Hinge/Corepressor Binding) Domain; a LBD or AF-2 or E (Ligand-Binding/Transcriptional Activation) Domain; and a variable F (Carboxyl-Terminal) Domain. In general, the RARs contain six regions from A-F. The DBD binds to the RARE (Retinoic Acid Response Element) region in the DNA. The RAREs consists of a DRs (Direct Repeats) of AGG/TTCA motif with a spacer region of (n)25. Vitamin-A in the liver is converted to all-trans-Retinoic Acid, diffuses easily to the target tissues through cellular membranes and is translocated to the RARs through CRABP (Cellular Retinoic Acid Binding Protein) (Ref.1 & 2).
The mechanism of all-trans-Retinoic Acid-induced Apoptosis is through Mitochondrial Dysfunction involving TRAIL (TNF-Related Apoptosis-Inducing Ligand) and it’s Death Receptors, the TRAILRs (TNF-Related Apoptosis-Inducing Ligand Receptors). all-trans-Retinoic Acid activate Ifns (Interferons) and both function synergistically to activate TRAILRs and Caspase8 (Cysteine Aspartate Specific Protease-8) that in turn induce the mitochondrial damage leading to the release of CytoC (Cytochrome-C). The TRAILRs contain the functional DDs (Death Domains), capable of inducing Apoptosis. Binding of TRAIL to TRAILRs and subsequent all-trans-Retinoic Acid-mediated activation leads to the recruitment of the Apoptosis Regulator FADD (Fas-Associated via Death Domain), which functions as a molecular bridge to Caspase8. Upon activation the TRAILRs indirectly bind to FADD via the GTP-binding protein DAP3 (Death-Associated Protein-3). Caspase8 cleaves BID (BH3 Interacting Domain Death Agonist) and the tBID (Truncated BID) translocates to mitochondria, inducing CytoC release. CytoC in association with APAF1 (Apoptotic Protease Activating Factor-1) activates Caspase9 (Apoptosis Related Cysteine Protease-9). Caspase9, in turn, causes the cleavage of proteins required for cellular viability, resulting in Apoptosis. Caspase9 also activates Caspase3 which directly cleaves downstream substrates like PARPs (Poly (ADP-Ribose) Polymerases) (Ref.3). Apoptosis by TRAIL and TRAILRs is controlled by FLIP (FLICE Inhibitory Protein), which inhibits the activation of Caspase8. Another mechanism of all-trans-Retinoic Acid induced Apoptosis requires Cytokine-mediated stimulation of PLA2 activity, resulting in the generation of excess Arachidonic Acid and this pathway is chiefly functional in the brain cells. Retinoic Acid functions as an important regulatory signaling molecule for Cell Growth, Differentiation and Neurodegeneration both during embryogenesis and in adult stage. Retinoic Acid induced Apoptosis through Death Receptors is a potentially promising approach for treatments of diseases like Schizophrenia, Alzheimer Disease and also for Cancer therapy (Ref.4 & 5).
References
- 1
- Lee GS, Kochhar DM, Collins MD. Retinoid-induced limb malformations.
- 2
- Miano JM, Berk BC. Retinoids: versatile biological response modifiers of vascular smooth muscle phenotype.
- 3
- Farooqui AA, Antony P, Ong WY, Horrocks LA, Freysz L. Retinoic acid-mediated phospholipase A2 signaling in the nucleus.
- 4
- Gottlieb RA. Programmed cell death.
- 5
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency.
 关于我们
关于我们
