Remodeling_of_Adherens_Junctions
发布时间:2019-12-11 11:42 来源:SABiosciences
- 通路
- 概述
Review
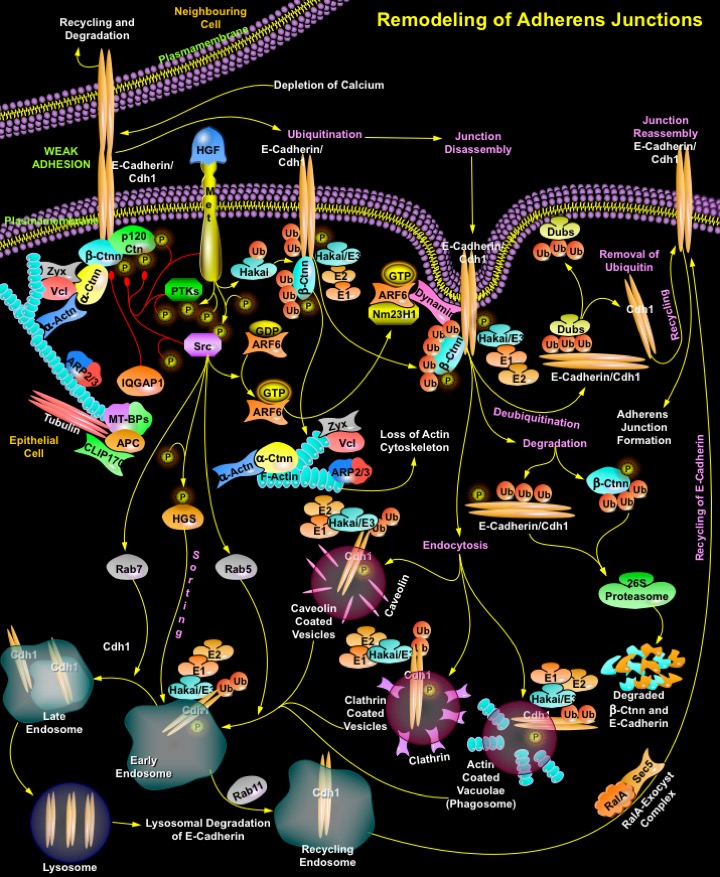
The intercellular Adherens Junctions (AJs) are specialized sub-apical structures that function as principle mediators of cell-cell adhesion. Their disassembly correlates with a loss of cell-cell contact and an acquisition of migratory potential. The Adherens Junctions have a crucial role both as sensors of extracellular stimuli and in regulating the dynamics of epithelial cell sheets or with neighboring cells. Cadherins, the Type-I transmembrane proteins of the Adherens Junctions, are principally responsible for homotypic cell-cell adhesion. E-Cadherin, which is present primarily in epithelia, is the best-characterized Cadherin and represents the prototype of classical Cadherins. The extracellular domain of E-Cadherin binds to Ca2+ (Calcium) and forms complexes with the extracellular domains of E-Cadherin molecules on neighboring cells. The cytoplasmic domain of E-Cadherin associates with cytosolic proteins called Catenins (Alpha, Beta and p120), which in turn provide anchorage to the Actin cytoskeleton to form stable cell-cell contacts. Ctnn-Beta (Catenin-Beta) binds through its armadillo repeats to the distal region of the E-Cadherin tail, thereby stabilizing the E-Cadherin molecule and facilitating transport of the newly synthesized protein to the cell surface. Ctnn-Alpha (Catenin-Alpha) binds to Ctnn-Beta and links components of the Adherens Junctions to the Actin cytoskeleton. It also binds to Vcl (Vinculin), Zyx (Zyxin) and Alpha-Actn (Alpha-Actinin), which in turn binds to F-Actin. p120Ctn (p120Catenin), another member of the Catenin family, binds to the juxtamembrane region of E-Cadherin and stabilizes Cadherin molecules at the cell surface. Additional proteins and regulators of Actin polymerization such as the Actin-related protein, ARP2/3 complex also occur at the Adherens Junctions (Ref.1 & 2). However, the Cadherin-Catenin mediated cell-cell adhesion is regulated by IQGAP1 (IQ Motif Containing GTPase Activating Protein-1), both positively and negatively. IQGAP1 captures and stabilizes Microtubules/Tubulins through the Mt-BPs (Microtubule-Plus-End-Binding Proteins), APC (Adenomatous Polyposis Coli) and CLIP170 (Cytoplasmic Linker Protein-170Alpha-2), leading to establishment of polarized cell morphology and directional cell migration. But localization of IQGAP1 to sites of cell-cell contact and activation by APC/CLIP70/Mt-BPs reduces E-Cadherin-mediated cell-cell adhesion by interacting with Beta-Ctnn, causing the dissociation of Alpha-Ctnn from the Cadherin-Catenin complex and formation of weak adhesions. The level of junction proteins at the site of cell-cell contacts is modulated by transcriptional regulation and/or protein degradation through the Ubiquitin-Proteasome pathway. Apart from transcriptional regulation endocytosis and recycling of junction proteins is an alternative mechanism allowing cells to undergo rapid changes in morphology in response to extracellular stimuli (Ref.3).
Extracellular stimuli like depleted Ca2+ levels and growth factors like HGF (Hepatocyte Growth Factor) are major factor that significantly increase the process of endocytosis and recycling of cell surface E-Cadherin. Endocytosis not only plays a role in dynamics, its effect on the regulation of Adherens Junctions dynamics is well-documented. E-Cadherins are rapidly removed from the plasma membrane; subsequently, they are recycled to sites of new cell-cell contacts. Receptor and non-receptor tyrosine kinases, such as Met and Src (v-Src Avian Sacroma (Schmidt-Ruppin A-2) Viral Oncogene), respectively, along with other PTKs (Protein Tyrosine Kinases) have an active function in this process, as they phosphorylate tyrosine residues in the short intra-cytoplasmic tail of E-Cadherins, thereby promoting their internalization by endocytosis. Tyrosine Kinases also phosphorylate Ctnn-Beta and p120Ctn causing disassociation of cytoskeletal proteins from junctions and thereby weakening junction morphology (Ref.4). The underlying molecular mechanism reveals that Hakai, an E3-Ubiquitin ligase mediates ubiquitination of the E-Cadherin complex and induces its endocytosis. It binds to tyrosine phosphorylated E-Cadherins and facilitates the internalization and subsequent Ubiquitin-dependent degradation of E-Cadherins. E-Cadherin bound to Hakai may initiate the activation of intracellular signaling pathways, whereas the E3-ligase function of Hakai mediates the transfer of Ubiquitin chains to E-Cadherin and Ctnn-Beta through the E1-E2 ubiquitination system. The process of ubiquitination involves Ubiquitin itself, the Ubiquitin-activating enzyme (E1), Ubiquitin conjugating enzymes (E2), and a variety of Ubiquitin ligases (E3). Ubiquitinated Ctnn-Beta and E-Cadherin is degraded in the 26S Proteasome complex. The ubiquitinated E-Cadherin may get degraded into short peptides by Proteasome with the release of Ubiquitin chain, or undergo deubiquitination by Dubs (Deubiquitinating Enzymes). Dubs act on the Ubiquitin chain and cleave the Ubiquitin chain from that protein, resulting in rescuing the protein from degradation. E-Cadherin is then recycled back to the cell surface, which then facilitates the reassembly of cell junctions. The Ubiquitin in turn is released and recycled. But ubiquitinated E-Cadherin-Hakai complexes are internalized via different endocytic structures including Caveolin-coated vesicle, Clathrin-coated vesicle and Actin-coated vacuolae (Phagosome), which are rapidly uncoated and fuse to early endosomes. Subsequently they are delivered to either late endosome which targets for degradation in lysosome or recycling endosome for channeling back to the cell surface (Ref.4 & 5).
The modification of E-Cadherin by Ubiquitin is essential for its sorting to the lysosome, which occurs by a process mediated by Src phosphorylated HGS (Hepatocyte Growth Factor-Regulated Tyrosine Kinase Substrate) and activated Rab GTPases. The intracellular trafficking of E-Cadherin is mediated by the activation of Rab family of GTPases that includes Src activated Rab5 and Rab7. Rab5 and Rab7 actively function during formation of early and late endosomes, respectively. Several other small GTPases such as Ras and Rab family members are also involved directly and indirectly in the endocytosis and recycling of E-Cadherin in several epithelial cells. For instance, the activation of ARF6 (ADP-Ribosylation Factor-6) by Src promotes the Clathrin-dependent internalization of E-Cadherin, resulting in the disassembly of Adherens Junctions without the remodeling of Actin filament. ARF6-GTP recruits NM23H1 (Nonmetastatic Protein-23 Homolog-1), a nucleoside diphosphate kinase, to facilitate Dynamin-mediated endocytosis during Adherens Junctions remodeling. Similarly Rab11 GTPase directs the entry of E-Cadherin to recycling endosomes resulting in junction remodeling. RalA (v-Ral Simian Leukemia Viral Oncogene Homolog-A) and ExoC2 (Exocyst Complex Component-2) further enhance E-Cadherin recycling to the lateral membrane where it initiates junction signaling. Thus ARF6-GTP specifies the delivery and insertion of recycling endosomal membranes to regions of dynamic plasma membrane remodeling through its interaction with the vesicle-tethering exocyst complex (Ref.6 & 7). Hence to maintain the dynamics of epithelial monolayers, E-Cadherin is rapidly removed from the plasma membrane and then subsequently recycled back to the cell surface to reform new cell-cell contacts. Recycling of E-Cadherin through the endosomal recycling pathway represents an effective mechanism for the remodeling of adhesive contacts in dynamic situations where cell-cell contacts must be dissolved and reformed. These have opened new avenues for investigating cell-cell adhesion during development and in disease states such as tumor metastasis (Ref.1).
References
- 1
- D\'Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do.
- 2
- Kowalczyk AP, Reynolds AB. Protecting your tail: regulation of cadherin degradation by p120-catenin.
- 3
- Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration.
- 4
- Palacios F, Tushir JS, Fujita Y, D\'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions.
- 5
- Pece S, Gutkind JS. E-cadherin and Hakai: signalling, remodeling or destruction?
- 6
- Palacios F, Price L, Schweitzer J, Collard JG, D\'Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration.
- 7
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function.
 关于我们
关于我们
