Ras_Pathway
发布时间:2019-12-11 11:39 来源:SABiosciences
- 通路
- 概述
Review
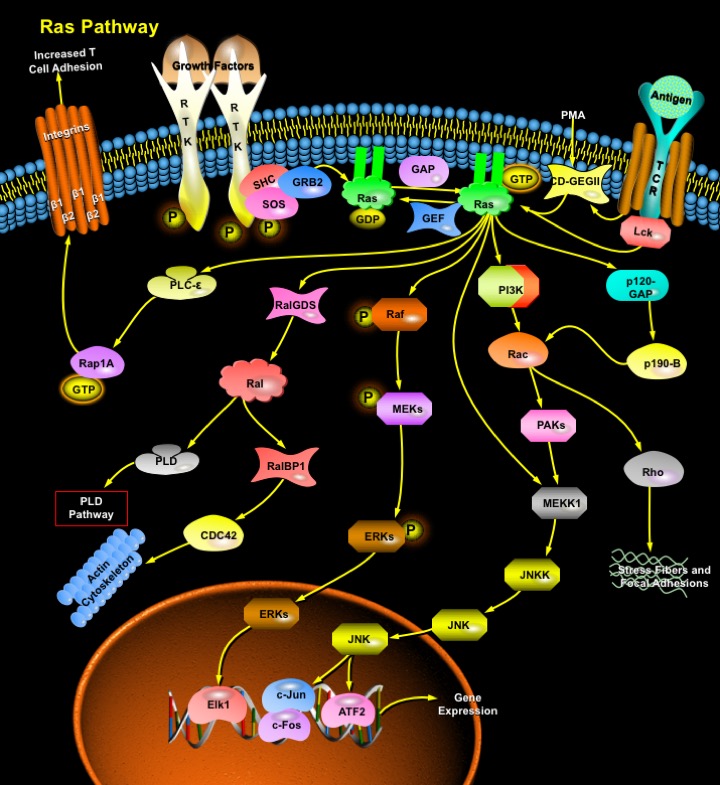
Ras is a membrane-associated guanine nucleotide-binding protein that is normally activated in response to the binding of extracellular signals, such as growth factors, RTKs (Receptor Tyrosine Kinases), TCR (T-Cell Receptors) and PMA (Phorbol-12 Myristate-13 Acetate). Ras signaling affects many cellular functions, which includes cell proliferation, apoptosis, migration, fate specification, and differentiation. Ras acts as a binary signal switch cycling between ON and OFF states, which are characterized in terms of a small molecule, a guanine nucleotide, bound to the protein. In the resting cell, Ras is tightly bound to GDP (Guanosine Diphosphate), which is exchanged for GTP (Guanosine Triphosphate) upon binding of extracellular stimuli to cell membrane receptors. In the GTP-bound form, Ras interacts specifically with so-called effector proteins, thereby initiating cascades of protein-protein interactions that may finally lead to cell proliferation. To return to the inactive OFF state, Ras cleaves off the terminal phosphate moiety, the Gamma-Phosphate, of GTP in an enzymatic process, the intrinsic GTPase reaction. The remaining GDP-bound Ras is no longer able to interact with effectors, it is switched OFF. Ras is thought to activate a number of signaling pathways, including the Raf/MEK/ERK (Extracellular Signal-Regulated Kinases) pathway, the MEKK/SEK/JNK (Jun N-terminal Kinases) pathway, a PI3K (Phosphatidylinositol 3-Kinase)/Akt/NF-KappaB (Nuclear Factor-Kappa B) pathway, a p120-GAP/p190-B/Rac/NF-KappaB pathway, and a Raf/MEKK1/IKK (I-KappaB Kinase)/I-KappaB/NF-KappaB pathway.
Most growth factors that signal through RTKs or heterotrimeric GPCR (G-Protein Coupled Receptors) stimulate Ras by recruiting the GEF (Guanine-Nucleotide Exchange Factor) SOS (Son of Sevenless) to the membrane. SOS exists in a complex with the adapter protein GRB2 (Growth Factor Receptor-Bound Protein-2). Upon receptor activation, the GRB2/SOS complex is translocated to the membrane by binding of GRB2 to tyrosyl-phosphorylated residues in RTKs or additional adapter proteins. GTP-bound Ras recruits and activates Raf. Raf initiates a cascade of protein phosphorylation by first phosphorylating MEKs. Phosphorylated MEK in turn phosphorylates ERKs. Phosphorylated ERK moves from the cytoplasm into the nucleus where it subsequently phosphorylates a number of transcription factors, including the specific transcription factor called Elk1. Phosphorylated transcription factors turn on transcription (gene expression) of specific sets of target genes. The activity of Ras is limited by the hydrolysis of GTP back to GDP by GAP (GTPase Activating Proteins) (Ref.1).
A second effector is PI3K, which synthesizes several lipid second messengers that activate small G proteins such as Rac and CDC42 (Cell Division Cycle-42). Rac also has multiple effectors, one of which is the serine threonine kinase PAKs (p21 Activated Kinases). The PI3K-mediated survival signal is also triggered by the activation of Akt/PKB (Protein Kinase-B). The third and most recently established effector is the Ral guanine nucleotide exchange factor, RalGDS (Ral-Guanine Nucleotide Dissociation Stimulator). RalGDS is a GEF (Guanine Nucleotide Exchange Factor) for the small GTPase Ral. Currently, five Ral GEFs have been identified; RalGDS, Rgl, Rlf, Rgr and RalGEF2 that are direct targets for Ras (Ref.2). They provide a mechanism for Ral activation by extracellular signals via a variety of receptors, including GPCR and RTKs.
Reactive free radicals and cellular redox stress have been proposed to directly activate Ras. NO (Nitric Oxide) promotes the direct post translational modification of Ras by single S-nitrosylation at Cys118. These results in stimulation of guanine nucleotide exchange, possibly by destabilization associates with other effectors, leading to transduction of Ras mediated signals through multiple pathways. In addition to Raf, PI3K and RalGDS other Ras effectors have been proposed, including p120GAP, PKC-zeta, Rin1, AF6, and NF1 GAP (Ref.3). TCR engagement also leads to the activation of Ras via a signaling pathway involving the activation of p56 (Lck) and PKC (Protein Kinase-C). Biochemical and genetic studies have now confirmed the functional relevance of Ras effectors. The Raf protein kinase family controls the activation of the MAPK (Mitogen-Activated Protein Kinases) pathway and plays a major role in controlling proliferation and differentiation. The PI3K mediates some of the Ras-dependent actin cytoskeleton remodeling and protection against apoptosis. The third bonafide Ras effector is RalGDS, regulates multiple processes including receptor endocytosis, cytoskeletal changes, and DNA synthesis. The Ras-Raf-MEK-ERK pathway features several oncogenes and is deregulated in approximately 30% of all human cancers. It has also emerged as a prime target for antitumor therapy (Ref.4).
References
- 1
- Gay B, Suarez S, Caravatti G, Furet P, Meyer T, Schoepfer J. Selective GRB2 SH2 inhibitors as anti-Ras therapy.
- 2
- Emel Okan et al. The small-GTPase RelA activates transcription of the urokinase plasminogen activator receptor (uPAR) gene via an AP1-dependent mechanism.
- 3
- Diaz-Meco MT, Lozano J, Municio MM, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta.
- 4
- Kolch W. Ras/Raf signalling and emerging pharmacotherapeutic targets.
 关于我们
关于我们
