PAK_Pathway
发布时间:2019-12-11 11:08 来源:SABiosciences
- 通路
- 概述
Review
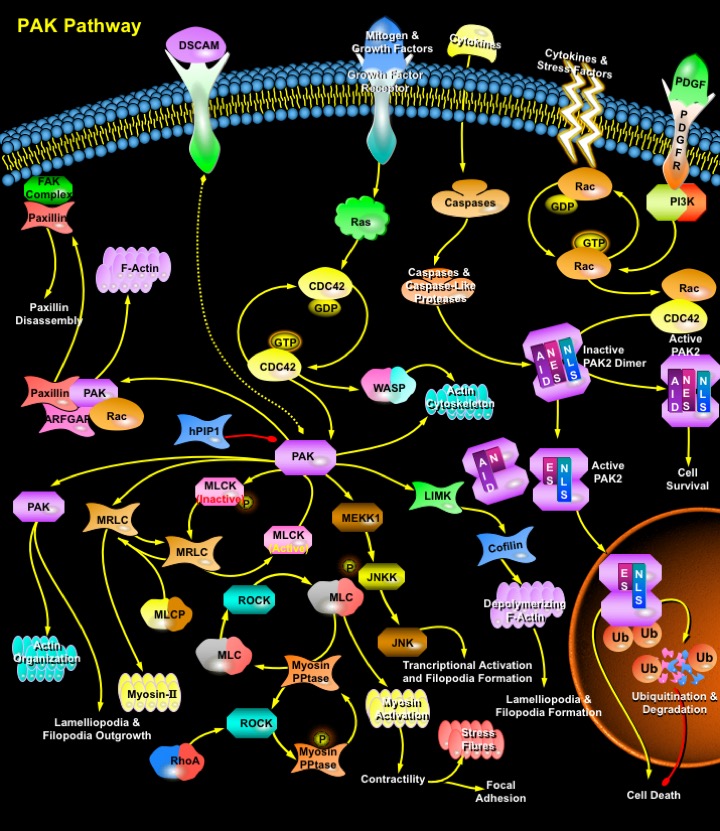
PAKs (p21-Activated Protein Kinases) are a growing family of serine/threonine protein kinases, which are activated in response to extracellular signals and regulate cell shape and motility. PAKs regulate diverse cellular functions, including gene expression, cytoskeletal actin assembly, MAPK (Mitogen-Activated Protein Kinase) pathways, neurite outgrowth, cell cycle control, and cell apoptosis (Ref.1). The mammalian PAK family consists of six members, which can be divided into two subfamilies according to sequence homology. The first subfamily consists of PAK1 (Alpha-PAK), PAK2 (Gamma-PAK, PAK I) and PAK3 (Beta-PAK). PAK1 and PAK3 are tissue-specific with the highest levels in brain, while PAK2 is ubiquitous. The second subfamily consists of the more recently identified PAK4, PAK5, and PAK6. Alpha-PAK is activated by growth factors such as PDGF (Platelet-Derived Growth Factor) and EGF (Epidermal Growth Factor) and by insulin. Alpha-PAK participates in the control of actin cytoskeletal dynamics. In contrast, Gamma-PAK is activated in response to stimuli leading to cytostasis, such as hyperosmolarity and DNA damage induced by ionizing and UV radiation or chemotherapeutic drugs. In addition, Gamma-PAK (but not Alpha- or Beta-PAK) is activated in response to apoptotic stimuli, such as anti-Fas, ceramide, or TNF (Tumor Necrosis Factor), and by Caspase cleavage followed by autophosphorylation. Expression of the catalytic domain of Gamma-PAK induces some of the morphological and biochemical changes characteristic of programmed cell death (Ref.2).
PAKs have been named according to their activation by the monomeric, p21 G-proteins CDC42 (Cell Division Cycle-42) and Rac. The GTP-bound forms of CDC42 and Rac regulate assembly of the actin cytoskeleton, in part by stimulation of PAKs and in part by activation of the intermediate switch proteins, WASP (Wiskott-Aldrich Syndrome Protein) and N-WASP. PAKs become activated by interaction with Rho-type small GTP-binding proteins Rac and CDC42 in the GTP-bound conformation, thereby relieving the inhibition of the RD (Regulatory Domain) on the CD (Catalytic Domain). These p21 G-proteins in the GTP-bound state bind to a conserved region within the N-terminal RD of PAKs and release block of the catalytic site by an overlapping AID (Auto-Inhibitory Domain). One way in which PAKs can influence actin organization and cell polarity is through phosphorylation of substrates such as MLCK (Myosin Light Chain Kinase) and myosin itself (Ref.3). PAKs can respond to receptor-mediated signals that direct their recruitment to the plasma membrane and subsequent activation. Although the ability of CDC42 and Rac GTPases to activate PAK is well established, relatively little is known about the negative regulation of PAK or the identity of PAK cellular targets. Interaction of hPIP1 (Human PAK1-Interacting Protein) with PAK1 inhibits the CDC42/Rac-stimulated kinase activity through the N-terminal regulatory domains of PAK1. hPIP1 is a negative regulator of PAK and PAK signaling pathways (Ref.4). PAKs also activate MAP (Mitogen Activated Protein) kinase cascades in vertebrates and in yeast. Expression of constitutively active Rac or CDC42 activates the JNKs (Jun N-terminal Kinases) and p38 but not the ERKs (Extracellular Signal Regulated Kinases). Indirect signals to PAK include Raf (because it cooperates with RacV12H40, RasV12G37, and RhoV14), RalGDS, Rin (both of which bind RasV12G37), and Ras-GAP (which can regulate the cytoskeleton through Rho). Rho kinase (p160ROCK) is another candidate because it binds Rho, Rac, and RacV12C40. PAKs have been reported to activate the JNK and NF-KappaB (Nuclear Factor-KappaB) pathways under different conditions. Activation of JNK causes phosphorylation and activation of several transcription factors, including c-Jun, ATF2 (Activating Transcription Factor-2) and Elk1. All of these transcription factors have been implicated in the expression of genes that regulate cell growth (Ref.5).
In response to tyrosine phosphorylation, adaptor protein Nck (Neuronal CDK) can also recruit PAK1 to the membrane to increase its activity and specificity. The PAK1 homologue Ste20 is involved in transmitting the mating-pheromone signal from the G-Beta Gamma subunits of a heterotrimeric G-Protein to a downstream MAPK cascade. Binding of G-Beta with the noncatalytic C-terminal region of Ste20 is essential for the activation of the MAPK cascade in signaling from the mating factor receptor. Recently, a family of PIXs (PAK-Interacting Exchange Factors) for Rac and CDC42 were identified as binding tightly to the fourth proline-rich domain in the N terminus of PAK. PIX can regulate PAK activity both by catalyzing GTP exchange on CDC42/Rac and by direct binding to PAK (Ref.6). Paxillin, a focal adhesion adaptor protein acts as a mediator of p21 GTPase-regulated actin cytoskeletal reorganization through the recruitment to nascent focal adhesion structures of an active PAK/PIX complex potentially via interactions with p95PKL (Paxillin-Kinase Linker). In contrast to activation of PAK2 by Rac or CDC42, cleavage and activation of PAK2 by Caspases or Caspase-like proteases correlates with programmed cell death and appears to be involved in the execution of programmed cell death. Proteolytic cleavage removes most of the N-terminal RD including the AID and generates constitutively active PAK2p34, a 34 kDa C-terminal fragment which contains the entire CD. Therefore, PAK2 appears to be unique among the PAK isoforms, because it can stimulate cell survival or induce cell death depending on the mechanism of activation (Ref.7).
Recently, Alpha-PAK has been shown to mediate signals from Ras through PI3K (Phosphoinositide 3-Kinase) and Akt to sustain cell transformation. Activated Akt stimulates Alpha-PAK activity through a p21 G-protein-independent mechanism. One of the downstream targets of Akt is the pro-apoptotic BCL2 (B-Cell Leukaemia-2) family protein BAD (BCL2 Associated Death Promoters). BAD dimerizes with anti-apoptotic BCL2 or BCLXL and inhibits their ability to block the release of CytoC (Cytochrome-C) from mitochondria. Phosphorylation of BAD at Ser-112 and Ser-136 results in dissociation from BCL2 or BCLXL and association with 14-3-3. Alpha-PAK has been shown to phosphorylate BAD at Ser-112 and Ser-136, and phosphorylation at these residues correlates with stimulation of cell survival (Ref.8). Stimulation of cell growth and cell survival by activated full-length PAK2 appears to be involved in the development of human cancer. Hyperactive PAK2 is present in several surgical human breast and colon cancer samples and in highly proliferative human breast cancer cell lines.
References
- 1
- Yi Tang, Sunil Marwaha, J. Lynn Rutkowski, Gihan I. Tennekoon, Peter C. Phillips, and Jeffrey Field. A role for Pak protein kinases in Schwann cell transformation.
- 2
- Joan Roig, Polygena T. Tuazon, Patricia A. Zipfel, Ann Marie Pendergast, and Jolinda A. Traugh Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK
- 3
- Luraynne C. Sanders, Fumio Matsumura, Gary M. Bokoch, Primal de Lanerolle Inhibition of myosin light chain kinase by p21-activated kinase.
- 4
- Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1.
- 5
- Scott T. Eblen,Jill K. Slack, Michael J. Weber, and Andrew D. Catling Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes.
- 6
- Zhou-shen Zhao, Edward Manser, Tsui-Han Loo,and Louis Lim. Coupling of PAK-Interacting Exchange Factor PIX to GIT1 Promotes Focal Complex Disassembly
- 7
- Gretel Buchwald, Eva Hostinova, Markus G. Rudolph, Astrid Kraemer, Albert Sickmann, Helmut E. Meyer,Klaus Scheffzek,and Alfred Wittinghofer. Conformational Switch and Role of Phosphorylation in PAK Activation
- 8
- Schürmann, A. F. Mooney,L. C. Sanders, M. A. Sells, H. G. Wang, J. C. Reed, and G. M. Bokoch. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis
 关于我们
关于我们
