P38_Signaling
发布时间:2019-12-11 11:00 来源:SABiosciences
- 通路
- 概述
Review
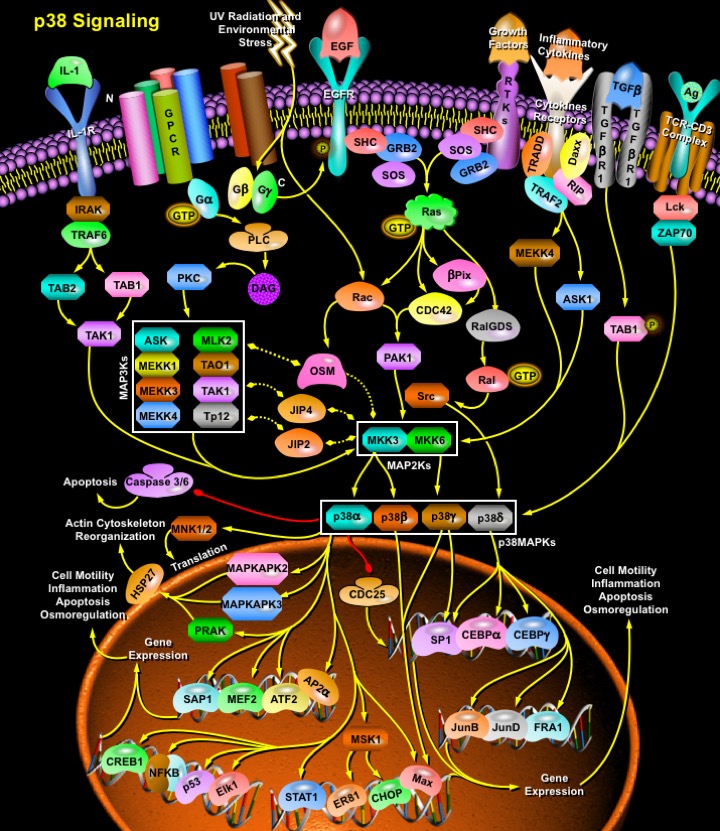
Cellular responses to many external stimuli involve the activation of several types of MAPK (Mitogen-Activated Protein Kinase) signaling pathways. MAPKs are a family of Serine/threonine kinases that comprise 3 major subgroups, namely, ERK (Extracellular signal–Regulated Kinase), p38 MAPK and JNK (c-Jun N-terminal Kinases). Despite the diversity in function and upstream signaling events, MAPKs are always activated by a highly conserved mechanism that involves phosphorylation on both a Thr (Threonine) and a Tyr (Tyrosine) residue catalyzed by a MAPK kinase. The phosphorylation motif Thr-Xaa-Tyr is located in the so called activation loop or T loop whose amino acid sequence varies among different MAPK subfamilies. Accordingly, there are different activating MAPK Kinases that in most cases are specific for each subgroup of MAPKs. p38 MAPKs belong to mammalian Stress-activated MAPK family. The p38 MAPK subfamily plays important roles in Cytokine production and the Stress response. Recent reports have also demonstrated additional functions for p38 MAPKs, for example, in the inhibition of Cell cycle progression, in developmental processes such as egg polarity and wing morphogenesis in Drosophila, and in the differentiation of several vertebrate cell types including Neurons, Adipocytes and Myoblasts. Four p38 MAPKs have been cloned so far in higher eukaryotes: p38-Alpha/XMpk2/CSBP, p38-Beta/p38-Beta22, p38-Gamma/SAPK3/ERK6, and p38-Delta/SAPK4. These four proteins are 60-70% identical in their Amino acid sequence and are all activated by MKK6 (MAPK Kinase-6). Another MAPK kinase, MKK3 (MAPK Kinase-3), has been shown to phosphorylate and activate p38-Alpha, p38-Gamma, and p38-Delta but not p38-Beta2. The mammalian p38 MAPK families are activated by cellular stress including UV irradiation, heat shock, high Osmotic stress, Lipopolysaccharide, Protein synthesis inhibitors, Proinflammatory cytokines (such as IL-1 (Interleukin-1) and TNF-Alpha (Tumor Necrosis Factor-Alpha)) and certain Mitogens (Ref.1 & 2).
Growth factors play an important role in the activation of p38 MAPKs. The binding of a peptide such as EGF (Epidermal Growth Factor) or Insulin or other Growth Factors to the RTKs (Receptor Tyrosine Kinase) causes them to dimerize, and this activates their kinase activities, leading to their autophosphorylation. This phosphorylation produces binding sites for proteins with SH2 (Src Homology-2) domains, such as GRB2 (Growth Factor Receptor Bound Protein-2). GRB2, with SOS (Son of Sevenless protein) bound to it, binds to the RTK, which activates SOS. SOS is a GEF (Guanine nucleotide Exchange Factor) which activates Ras by inducing it to release GDP and exchange it for GTP. GAPs (GTPase Activating Proteins) accelerate the intrinsic GTP hydrolytic activity of Ras, thereby promoting the formation of the inactive, GDP-bound form of Ras. Active Ras is able to stimulate many effector proteins including p120-GAP, GEFs such as RalGDS (Ral Guanine Nucleotide Dissociation Stimulator), Beta-PIX (Beta-PAK (p21/ CDC42/ Rac1-Activated Kinase)-Interacting Exchange Factor), and a number of Protein Kinases such as PI3Ks (Phosphatidylinositol 3-Kinase), PKC (Protein Kinase-C) and Rho family GTPases Rac and CDC42 (Cell Division Cycle-42). RalGDS, Beta-PIX, Rac and CDC42 all activate p38 MAPKs. One of the target proteins for Rac and CDC42 is PAK (p21-Activated Kinase), which activates p38 by phosphorylating MKKs. Effect of Beta-PIX on p38 is exerted through the CDC42/Rac-PAK pathway and requires PAK Kinase activity. RalGDS, on the other hand activates p38MAPKs through Ral (v-Ral Simian Leukemia Viral Oncogene Homolog) and Src (v-Src Avian Sacroma (Schmidt-Ruppin A-2) Viral Oncogene) respectively. Recently, it has been found that TGF-Beta (Transforming Growth Factor-Beta) also activates p38. The molecular mechanism of TGF-Beta -induced activation of p38MAPK signaling are not defined. TAK1 (TGF-Beta-Activated Kinase-1) is believed to phosphorylate MKK3/6 in TGF-Beta signaling (Ref. 3 , 4 & 5).
Proinflammatory Cytokines such as IL (Interleukin) and TNF (Tumor Necrosis Factor) also stimulate p38 signaling. IL-1 (Interleukin-1) is a central regulator of the immune and inflammatory responses by which various inflammatory genes are induced. IL-1 signaling is known to involve PI3K, p38 MAPK and ERK. After IL-1 is bound to its receptor IL-1R (IL-1 Receptor), a complex is formed between the Type-1 Receptor and the Receptor Accessory Protein. The cytosolic proteins MyD88 (Myeloid Differentiation primary response gene-88) and TollIP (Toll-Interacting Protein) are recruited to this complex, where they function as Adaptors, recruiting IRAK1 (IL-1 Receptor-Associated Kinase-1) in turn. IRAK1, a Serine-threonine Kinase, activates and recruits TRAF6 (TNF Receptor-Associated Factors-6) to the IL-1 Receptor complex. Eventually, phosphorylated IRAK is ubiquitinated and degraded. TRAF6 signals through the TAB1 (TAK1 Binding Protein-1)/TAK1 (TGF-Beta-Activating Kinase-1) Kinases to activate MKKs, which further activates p38 MAPK. p38 is also activated by another Cytokine, TNF. Binding of the Receptor TNFR1 to TNF-Alpha results in conformational changes in the Receptor’s intracellular domain, resulting in rapid recruitment of several cytoplasmic death domain–containing adapter proteins via homophilic interaction with the death domain of the receptor. The first adaptor recruited to the clustered receptor is the TNFR–associated protein with death domain, which functions as a docking protein for several signaling molecules, such as FADD (Fas-Associated protein with Death Domain), TRADD (Tumor Necrosis Factor Receptor-1-Associated Death Domain Protein), Daxx, TRAF2 (TNFR–Associated Factor-2), and RIP (Receptor-Interacting Protein). RIP associates with TRAF2 to generate MEKK4 (MAP/ERK Kinase Kinase-4) and ASK1 (Apoptosis Signal-regulating Kinase-1). Both MEKK4 and ASK1 activate p38 MAPKs by activating MKK3 and MKK6 (Ref. 6 & 7).
Activation of p38 MAPK also occurs via GPCR (G-Protein Coupled Receptors) and cellular stresses. p38s are activated primarily by GPCR agonists acting through PKC. PKC activation occurs when GPCRs coupled to PLC (Phospholipase-C) are activated, releasing DAG (Diacylglycerol). The binding of hormones, growth factors, neurotransmitters, and other agonists of GPCR results in activation of PLC via a G-Protein-dependent phenomenon. Heterotrimeric G-Proteins consist of Alpha, Beta and Gamma subunits that are stably associated in the inactive, GDP-bound state. Physical interaction between a G-Protein and an agonist-occupied GPCR triggers the exchange of GDP for GTP on the Alpha subunit and the subsequent dissociation of this subunit from the tightly associated Beta Gamma dimer. Both the GN-Alpha-GTP and GN-Beta Gamma entities participate in PLC activation and PKC signaling. PKC when activated activates several MAP3Ks including ASK1, MEKK1, MEKK4, MLK2 and 3, DLK (Dual Leucine Zipper Kinase), TPL2 (Tumor Progression Locus-2), TAK1 and TAO1/TAO2. MAP3Ks activates MKK3 and MKK6, which further activates p38 MAPKs. Numerous physical and chemical stresses, including hormones, UV irradiation, ischemia, osmotic shock and heat shock can also activate p38 by activating CDC42 and Rac. In addition, oxidative stress and GPCR stimulation also trigger EGF receptor autophosphorylation and thus indirectly activates p38 (Ref.1 & 8). TCR (T-Cell Receptor) is also known to activate p38, inducing Ifn-Gamma (Interferon-Gamma) production, Type-1 T-Helper Cell differentiation or T-Cell proliferation. TCR-induced p38 activation does not rely on the classic MAPK cascade. Activation through the TCR results in the sequential activation of Lck (Lymphocyte-Specific Protein-Tyrosine Kinase) and ZAP70 (Zeta-Chain-Associated Protein Kinase). Once it is activated by Lck, ZAP70 phosphorylates p38 on Tyr323, leading to p38 autophosphorylation and activation. Following its activation, p38 translocates to the nucleus and directly or indirectly activates multiple downstream effector pathways to generate biologic responses. p38 MAPK downstream targets include several kinases, transcription factors and cytosolic proteins. Among the Kinases that are activated by p38 MAP kinase are MAPKAPK2 (MAPK-Activated Protein Kinase-2), MAPKAPK3 (MAPK-Activated Protein Kinase-3), PRAK (p38-related/activated protein kinase), MSK1 (Mitogen- and Stress-Activated Protein Kinase-1) and MNK1/2 (MAP kinase-interacting kinase 1/2). The kinase MAPKAPK2 is perhaps the best characterized p38 MAP kinase substrate. Together with MAPKAPK3, it belongs to the RSK family of serine-threonine kinases and was the first kinase substrate found to be activated by the p38MAPK. Upon its phosphorylation/activation by p38, MAPKAPK2 phosphorylates and activates in turn various substrates, including the small HSP27 (Heat Shock Protein-27), CREB (cAMP Response Element-Binding Protein), and ATF1 (Activating Transcription Factor-1). MNK1 can also serve as a substrate for p38 MAP kinase. MNK1 can regulate cap-dependent gene translation through phosphorylation of the eIF4E (eukaryotic Initiation Factor of Translation-4E). Similarly, the kinases MSK1 and MSK2 can be activated p38 MAP kinase pathway, and activation of both pathways is required for generation of MSK1 and MSK2 kinase activities in response to either stress or growth factors. Upon activation, MSK1 and MSK2 activate CREB, the HMG14 (High-Mobility-Group protein-13) and Histone H3 and ER81, thus regulating basal and early gene transcription (Ref. 9 & 10).
The activation of p38 MAP kinase can also directly influence gene transcription, as a growing number of transcription factors are known to be direct targets of p38. Direct phosphorylation and activation have been described for ATF1, ATF2, and ATF-6, the MEF2A/C (Myocyte Enhance Factor-2A/C), SAP1A (Signaling lymphocytic Activation molecule associated Protein-1A) and the Elk1 (ETS-domain transcription factor-1). In addition, NFAT (Nuclear Factor of Activated T cells), a ubiquitous regulator of cell differentiation and a critical transcription factor in early embryonic development, is also a target for p38. Another important target of the p38 MAP kinase is the tumor suppressor protein p53, whose activation is positively regulated by p38. Other transcription factors phosphorylated by p38 include CHOP (C/EBP-Homologous Protein), CREB, STAT1 (Signal Transducers and Activators of Transcription-1), NF-KappaB (Nuclear Factor-KappaB), Max/Myc complexes, SP1 and c/EBPs. Other substrates of p38 include AP2-Alpha1 (Adaptor-Related Protein Complex-2-Alpha-1 Subunit), JunB, JunD and FRA1 (Fos-Related Antigen-1), Caspases3/6 and CDC25 (Cell Division Cycle-25). The p38 MAPK modules are also linked to scaffold proteins including JIP2 (JNK-Interacting Protein-2), JIP4 (JNK-Interacting Protein-4) and the recently described OSM (Oncostatin-M) protein, which interacts with actin cytoskeleton. In general, p38 appears to play a major role in Apoptosis, Cytokine production, Transcriptional regulation, and Cytoskeletal reorganization, and has been causally implicated in Sepsis, Ischemic Heart disease, Arthritis, Human Immunodeficiency Virus infection, and Alzheimer's disease (Ref.11, 12 & 13).
References
- 1
- Mittelstadt PR, Salvador JM, Fornace AJ Jr, Ashwell JD. Activating p38 MAPK: new tricks for an old kinase.
- 2
- Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development.
- 3
- Vergarajauregui S, San Miguel A, Puertollano R. Activation of p38 Mitogen-Activated Protein Kinase Promotes Epidermal Growth Factor Receptor Internalization.
- 4
- Kaur R, Liu X, Gjoerup O, Zhang A, Yuan X, Balk SP, Schneider MC, Lu ML. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase.
- 5
- Xia W, Longaker MT, Yang GP. P38 MAP kinase mediates transforming growth factor-beta2 transcription in human keloid fibroblasts.
- 6
- Schiller M, Bohm M, Dennler S, Ehrchen JM, Mauviel A. Mitogen- and stress-activated protein kinase 1 is critical for interleukin-1-induced, CREB-mediated, c-fos gene expression in keratinocytes.
- 7
- Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway.
- 8
- Ammoun S, Lindholm D, Wootz H, Akerman KE, Kukkonen JP. G-protein-coupled OX1 OREXIN/hcrtr-1 hypocretin receptors induce caspase-dependent and-independent cell death through p38 mitogen-/stress-activated protein kinase.
- 9
- Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ Jr, Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases.
- 10
- Sudo T, Kawai K, Matsuzaki H, Osada H. p38 mitogen-activated protein kinase plays a key role in regulating MAPKAPK2 expression.
 关于我们
关于我们
