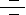Mitochondrial_Apoptosis
发布时间:2019-12-11 10:31 来源:SABiosciences
- 通路
- 概述
Review
Apoptosis is a naturally occurring process by which a cell is directed to Programmed Cell Death. Apoptosis is based on a genetic program that is an indispensable part of the development and function of an organism. In this process, cells that are no longer needed or that will be detrimental to an organism or tissue are disposed of in a neat and orderly manner; this prevents the development of an inflammatory response, which is often associated with Necrotic cell death. There are at least two broad pathways that lead to Apoptosis, an "Extrinsic" and an "Intrinsic" Pathway. In both pathways, signaling results in the activation of a family of Cys (Cysteine) Proteases, named Caspases that act in a proteolytic cascade to dismantle and remove the dying cell. The extrinsic pathway begins outside a cell, when conditions in the extracellular environment determine that a cell must die. The intrinsic apoptosis pathway begins when an injury occurs within the cell. The injury could result in necrosis and produce an inflammatory response, but the apoptotic machinery is in place to ensure that the damaged cell is packaged and removed cleanly, in order to prevent inflammation. Apoptosis in Mitochondria is the best known intrinsic apoptosis pathway (Ref.1 & 2).
The Mitochondrial pathway of apoptosis functions in response to various types of intracellular stress including Growth Factor withdrawal, DNA damage, unfolding stresses in the Endoplasmic reticulum and Death Receptor stimulation. Fas (CD95/Apo1), a member of the TNFR family, typify the classical view of DR (Death Receptor) function and play an important role in stimulating Mitochondrial apoptotic pathway. FasL (Fas Ligand), a homotrimeric protein acts as ligand for Fas and causes oligomerization of its Receptor on binding. Associated with this is the clustering of the Death Domains and binding of cofactor FADD (Fas-Associated via Death Domain). The FADD protein binds via its DED (Death Effector Domain) motif to a homologous motif in Procaspase8. The complex of Fas, FADD and ProCaspase8 is called the DISC (Death Inducing Signaling Complex). The cofactor function of FADD, in turn, is blocked by interaction with the regulator FLIP (FLICE Inhibitory Protein). Upon recruitment by FADD, Procaspase8 oligomerization drives its activation through self-cleavage. Active Caspase8 then activates downstream Caspases (Caspase3 and 7). Activated Caspase8 activates Caspase3 through two pathways. In the first pathway Caspase8 cleaves BID (Bcl2 Interacting Protein) and its COOH-terminal part translocates to Mitochondria where it triggers CytoC (Cytochrome-C) release. The released CytoC binds to APAF1 (Apoptotic Protease Activating Factor-1) together with dATP and Procaspase9 and activates Caspase9. The Caspase9 cleaves Procaspase3 and activates Caspase3. Another pathway is that Caspase8 cleaves Procaspase3 directly and activates it. Caspase 3 then commits the cell to Apoptosis. Besides DRs, Growth Factors also influence Mitochondrial Apoptosis via PI3K (Phosphatidylinositde-3 Kinase) and Akt (v-Akt Murine Thymoma Viral Oncogene Homolog) pathways. Growth Factors bind to Growth factor receptors and activate PI3K. Activation of PI3K pathways leads to Akt activation. Akt is very important in BAD (Bcl2-Antagonist of Cell Death) regulation, which is a proapoptotic member of Bcl2 (B-Cell Leukemia-2) family and is involved in Mitochondrial Apoptosis (Ref.6). PKC (Protein Kinase-C) may also play an important role in Apoptosis by activating p90RSKs (Ribosomal-S6 Kinases), which inhibits BAD (Ref. 3, 4 & 5).
In addition to Receptor-mediated Apoptosis, there is another pathway activated by various forms of Cellular Stress. Intrinsic stresses such as Oncogenes, direct DNA damage, Hypoxia, and Survival factor deprivation, can activate the Intrinsic Apoptotic pathway. p53 is a sensor of cellular stress and is a critical activator of the intrinsic pathway. The DNA checkpoints proteins, ATM (Ataxia Telangiectasia Mutated protein), and Chk2 (Checkpoints Factor-2) directly phosphorylate and stabilize p53 and inhibit MDM2 (Mouse Double Minute-2 Homolog) mediated ubiquitination of p53. MDM2 binds p53 and mediates the nuclear export. When bound to MDM2, p53 can no longer function as an activator of transcription. p53 initiates Apoptosis by transcriptionally activating proapoptotic Bcl2 family members and repressing antiapoptotic Bcl2 proteins and CIAPs (Cellular Inhibitor of Apoptosis). Other p53 targets include BAX (BCL2 Associated X-protein), Noxa, PUMA (p53-Upregulated Modulator of Apoptosis) and the most recently identified, BID. p53 also transactivates other genes that may contribute to Apoptosis including PTEN (Phosphatase and Tensin Homolog Deleted On Chromosome-10), APAF1, Perp, p53AIP1 (p53-regulated Apoptosis-Inducing Protein-1), and genes that lead to increases in ROS (Reactive Oxygen Species). These ROS lead to generalized oxidative damage to all Mitochondrial components. Damage to Mitochondrial DNA disrupts Mitochondrial oxidative phosphorylation, contributing to a number of Human diseases (Ref. 5 & 6).
Following the reception of stress signals, proapoptotic Bcl2 family proteins are activated and subsequently interact with and inactivate antiapoptotic Bcl2 proteins. Bcl2 family consists of both anti- and proapoptotic members that possess conserved alpha-helices with sequence conservation clustered in Bcl2 Homlogy domains. Proapoptotic members include "Multidomain" BAX family proteins such as BAX, BAK (Bcl2 Antagonist Killer) etc. that display sequence conservation in BH1-3 region and "BH3-only" proteins such as BID, BAD, Noxa, PUMA and BIM (BCL2-Interacting Protein) that have only the short BH3 motif. BAX family proteins act downstream in Mitochondrial disruption, whereas BH3-only proteins act upstream in the pathway, detecting developmental death cures or intracellular damages. Anti-apoptotic members like Bcl2, BclXL and their relatives exhibit homology in all segments BH1-4. The antiapoptotic and proapoptotic protein interaction leads to the destabilization of the Mitochondrial membrane and release of apoptotic factors including CytoC, SMAC (Second Mitochondria-Derived Activator of Caspase), Arts and Omi/HTRA2 (High Temperature Requirement Protein-A2). Bcl2L (Bcl2-Like), BCLXL and other anti-apoptotic BCL2 family members reside in the outer Mitochondrial membrane and prevent CytoC release. BAX , BID and BIM are initially inactive and must translocate to Mitochondria to induce apoptosis, either by forming pores in Mitochondria directly or by binding via BH3 domains to BCL2, BCLXL and BFL1, and antagonizing these anti-apoptotic proteins. MMP (Mitochondrial Membrane Permeabilization) is clearly a pivotal event in the progression of Apoptosis in many systems. At least two mechanisms of MMP have been described. First mechanism proposes that VDAC (Voltage-Dependent Anion Channel), ANT (Adenine Nucleotide Transporter), PBR (Peripheral-type Benzodiazepine Receptor) and CypD (Cyclophilin-D) come together to form the PTPC (Permeability Transition Pore Complex). This pore complex may also associate with BAX, BAK1 or BIM, which accelerate channel opening or BclXL, which causes closure. According to the second mechanism BAX is released from its interaction with 14-3-3 and translocates from cytosol to Mitochondria in response to diverse signals. Here it oligomerizes forming protein pores. Pore formation also takes place in conjunction with the BH3-only Bcl-2 family member BID upon proteolysis to tBID (Truncated BID). BAK1 is located at Mitochondria and has similar pore forming properties to BAX, via its oligomerization and association with tBID. BID is activated by Caspase8-induced cleavage during DR signaling, whereas BIM is released from its association with microtubules (Ref. 5 & 7).
Other proteins released from Damaged Mitochondria, SMAC (Second Mitochondria-Derived Activator of Caspase)/ Diablo, Arts and Omi/HTRA2 (High Temperature Requirement Protein-A2), counteract the effect of IAPs (Inhibitor of Apoptosis Proteins), which normally bind and prevent activation of Caspase3. The interaction between Bcl family members, IAPs, SMAC and Omi/HTRA2 is central to the intrinsic Apoptosis pathway. Recent studies demonstrated that another nuclease, EndoG (Endonuclease-G), is specifically activated by Apoptotic stimuli and is able to induce nucleosomal fragmentation of DNA independently of Caspase and DFF (DNA-Fragmentation Factor)/ CAD (Caspase-Activated DNAse). EndoG is a mitochondrion-specific nuclease that translocates to the nucleus and cleaves chromatin DNA during Apoptosis. Another protein, AIF (Apoptosis Inducing Factor) has also been attributed a role in Apoptosis, becoming active upon translocation from Mitochondria to nuclei, where it initiates chromatin condensation and large-scale DNA fragmentation (Ref.9). Endoplasmic reticulum stress also takes part in apoptosis by activating Caspase3 via Caspase12. Programmed cell death and its morphologic manifestation of Apoptosis is a conserved pathway that in its basic tenets appears operative in all metazoans. Apoptosis also operates in adult organisms to maintain normal cellular homeostasis. This is especially critical in long-lived mammals that must integrate multiple physiological as well as pathological death signals, which for example includes regulating the response to infectious agents. Gain and loss of function models of genes in the core Apoptotic pathway indicate that the violation of cellular homeostasis can be a primary pathogenic event that results in disease. Evidence indicates that insufficient Apoptosis can manifest as Cancer or Autoimmunity, while accelerated cell death is evident in Acute and Chronic Degenerative diseases, Immunodeficiency, and Infertility (Ref. 8 & 9).
References
- 1
- Wilk S. Teaching resources. Apoptosis.
- 2
- Czerski L, Nunez G. Apoptosome formation and caspase activation: is it different in the heart?
- 3
- Kim R, Emi M, Tanabe K Role of mitochondria as the gardens of cell death.
- 4
- Beisner DR, Ch\'en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8.
- 5
- Alladina SJ, Song JH, Davidge ST, Hao C, Easton AS. TRAIL-induced apoptosis in human vascular endothelium is regulated by phosphatidylinositol 3-kinase/Akt through the short form of cellular FLIP and Bcl-2.
- 6
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops.
- 7
- Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death.
- 8
- Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis.
- 9
- Rosenberg P. Mitochondrial dysfunction and heart disease.
 关于我们
关于我们