MAPK_Signaling
发布时间:2019-12-11 10:28 来源:SABiosciences
- 通路
- 概述
Review
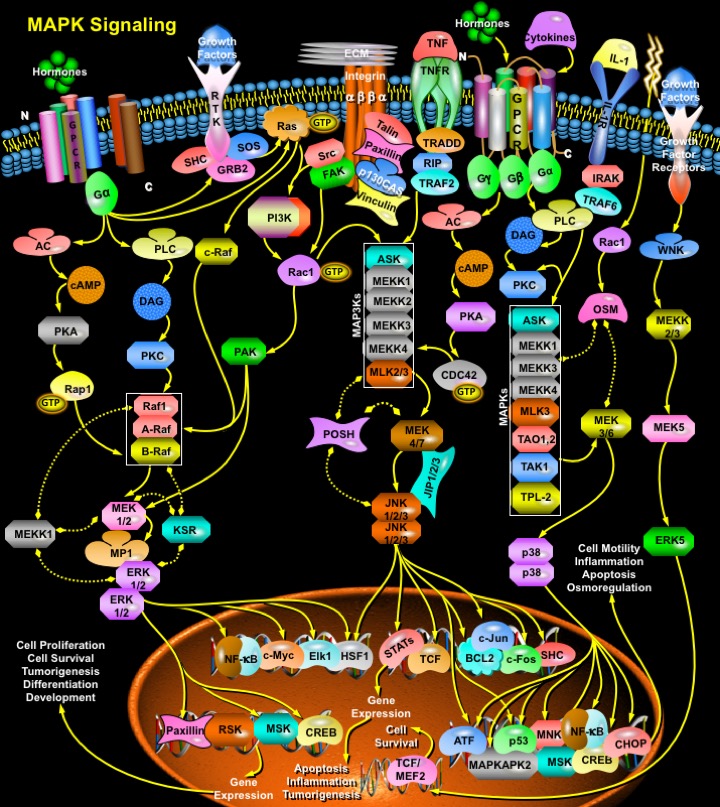
Intracellular signaling cascades are the main routes of communication between the Plasma membrane and regulatory targets in various intracellular compartments. Sequential activation of Kinases is a common mechanism of signal transduction in many cellular processes. During the past decade, several related intracellular signaling cascades have been elucidated, which are collectively known as MAPK (Mitogen-Activated Protein Kinase) signaling cascades. The MAPKs are a group of protein Serine/threonine Kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus. In combination with several other signaling pathways, they can differentially alter phosphorylation status of numerous proteins, including Transcription Factors, Cytoskeletal proteins, Kinases and other Enzymes, and greatly influence Gene Expression, Metabolism, Cell Division, Cell Morphology and Cell Survival. Furthermore, epigenetic aberrations of these enzymes or of the signaling cascades that regulate them have been implicated in a variety of human diseases including Cancer, Inflammation and Cardiovascular disease. There are four major groups of MAPKs in mammalian cells—the ERKs (Extracellular signal-Regulated Kinases), the p38MAPKs, the JNKs (c-Jun NH2-terminal Kinases) and the ERK5 (Extracellular signal-Regulated Kinase-5) or BMK cascades. These MAPKs are activated by dual phosphorylation at the tripeptide motif Thr-Xaa-Tyr. The sequence of this tripeptide motif is different in each group of MAPKs: ERK (Thr-Glu-Tyr); p38 (Thr-Gly-Tyr); and JNK (Thr-Pro-Tyr). Each MAPK pathway contains a three-tiered kinase cascade comprising a MAPKKK/MAP3K/MEKK/MKKK (MAP Kinase Kinase Kinase), a MAPKK/MAP2K/MEK/MKK (MAP Kinase Kinase) and the MAPK. This three-tier module mediates ultrasensitive switch-like responses to stimuli. Frequently, a MAPKKKK, MAP4K or MKKKK (MAPKKK Kinase) activates the MAPKKK. The MAPKKKs then phosphorylates a dual-specificity protein kinase MAPKK, which in turn phosphorylates the MAPK (Ref.1 & 2).
ERK, the most widely studied MAPK cascade, have been established as a major participant in the regulation of cell growth and differentiation, but when improperly activated contribute to malignant transformation. ERK1 and ERK2 form the central component in the ERK cascade. The ERK signaling cascade is activated by a wide variety of receptors involved in growth and differentiation including GPCRs (G-Protein Coupled Receptors), RTKs (Receptor Tyrosine Kinases), Integrins, and Ion Channels. A general activation scheme involves the activation of RTKs by Growth Factors, such as EGF (Epidermal Growth Factor). The subsequent auto-phosphorylation of the cytoplasmic tails of the receptor on tyrosine leads to the tyrosine phosphorylation of the adapter protein SHC. SHC can then recruit the GRB2 (Growth Factor Receptor Bound Protein-2)-SOS (Son of Sevenless protein) complex to the membrane via the SH2 domain of GRB2 binding to the phosphotyrosine on SHC. SOS, a GEF for Ras, can then exchange the GDP bound to Ras to GTP. Once Ras binds GTP, it can then recruit the Serine/threonine kinase Raf to the membrane. When Raf translocate to the membrane, it becomes activated and then phosphorylates the dual specificity kinases MKK1 and MKK2. The activated MKKs phosphorylate ERK1/ERK2 on Threonine183 and Tyrosine185 (at the TEY motif) (Ref.3). GPCR also play an important role in activation of ERKs. When the GPCR becomes activated by Ligands such as Neurotransmitters, Cytokines etc., it leads to the exchange of GDP for GTP on the GN-Alpha (Guanine Nucleotide-Binding Protein-Alpha) subunit. Upon activation, GN-AlphaI (Guanine Nucleotide-Binding Protein-Alpha-I) or GN-AlphaQ (Guanine Nucleotide-Binding Protein-Alpha-Q) subunits are separated from GN-Beta (Guanine Nucleotide-Binding Protein-Beta) and GN-Gamma (Guanine Nucleotide-Binding Protein-Gamma) subunits and are converted to their GTP bound states that exhibit distinctive regulatory features on the nine tmACs (Transmembrane Adenylate Cyclases) in order to regulate intracellular cAMP (Cyclic Adenosine 3',5'-monophosphate) levels. cAMP activate Rap1A (Ras-Related Protein-1A) and Rap1B (Ras-Related Protein Rap1B) through EPAC (Exchange Protein Activated by cAMP)-dependent pathway. cAMP activates cAMP-GEFI (cAMP-Regulated Guanine Nucleotide Exchange Factor-I)/EPAC1 and cAMP-GEFII (cAMP-Regulated Guanine Nucleotide Exchange Factor-II)/EPAC2 that in turn activate Rap1A and Rap1B, respectively. Rap1A and Rap1B then forms an active complex with BRaf (v-Raf Murine Sarcoma Viral Oncogene Homolog-B1) for MEK1/2 activation finally resulting in ERK1/2 activation. cAMP may also activate PKA (Protein Kinase-A), which may further activate Rap and thus BRaf. On the other hand, PKA also inactivates C-Raf. GN-Alpha also directly activates PLC (Phospholipase-C) which further activates PKC (Protein Kinase-C) via DAG (Diacylglycerol). PKC further activates Raf and thus ERK. A new mechanism has recently been identified that regulates MEK1-ERK interactions and is dependent on Rac and PAK (p21-Activated Kinase). Integrins also play an important role in regulating the efficiency of the RTK/Ras/ERK pathway. FAK (Focal Adhesion Kinase) is a major nonreceptor tyrosine kinase activated after Integrin-mediated adhesion to ECM (Extracellular Matrix) proteins such as FN (Fibronectin). Interaction between FAK and the cytoplasmic tail of Beta1 Integrins results in autophosphorylation of FAK tyrosine 397 (FAK pY397) that can lead to stimulation of a cell-signaling cascade that ultimately activates the Ras/MAPK/ERK pathway. In addition to FAK, members of the Src family of nonreceptor protein-tyrosine kinases also associate with Focal Adhesions and are involved in Integrin signaling. Interestingly, Src and FAK appear to function in association with each other as a result of the binding of the Src SH2 domain to an autophosphorylation site of FAK. Src then phosphorylates additional sites on FAK. Tyrosine phosphorylation of FAK creates binding sites for the SH2 domains of other downstream signaling molecules, including PI3K (Phosphatidylinositol 3-Kinase) and Rac. A key target of Rac is the protein-serine/threonine kinase PAK. Rac and CDC42 (Cell Division Cycle-42) can synergize with Raf to promote activation of the ERKs through mechanisms involving PAK1 phosphorylation of the MEK1 proline-rich sequence and PAK3 phosphorylation of Raf1. PAK3 can phosphorylate Raf1, enhancing Raf1 activation. Raf1 finally activates ERK1/2 via MEK1/2. ERK once activated translocates to the nucleus to phosphorylate and activate several nuclear targets. The major target of activated ERKs is RSK (90 kDa Ribosomal protein S6 Kinase). Active RSKs appear to play a major role in transcriptional regulation, translocating to the nucleus and phosphorylating such factors as the product of proto-oncogene c-Fos at Ser362, SRF (Serum Response Factor) at Ser103, and CREB (Cyclic AMP Response Element-Binding protein) at Ser133. ERK also translocates to the nucleus to phosphorylate transcription factor Elk1 (on Serine383 and Serine389). Another important target of ERK is NF-KappaB (Nuclear Factor-KappaB), which binds to its consensus sequence (5'-GGGACTTTC-3') and positively regulates the transcription of genes involved in immune and inflammatory responses, cell growth control, and apoptosis. Other nuclear targets of ERK include the MSKs (Mitogen- and Stress-activated protein Kinases), CREB, c-Myc, HSF1 (Heat-Shock Factor-1), Paxillin and many more transcription factors (Ref.4, 5 & 6).
Recently, another related kinase, ERK3, a nuclear protein kinase, has been cloned and is reported to exhibit about 50% homology to ERK1/ERK2 within its catalytic domain. However, it does not phosphorylate any typical ERK substrates. The phosphorylation site motif in the activation loop of ERK3 has a single phosphorylation site located at Serine189. Another member of ERK family is the ERK5 that contains at least ten consensus sites for MAPK phosphorylation and may be associated with keeping ERK5 in high active state. ERK5 can be activated by proliferative stimuli such as EGF, Serum, Lysophosphatidic acid, Neurotrophins and Phorbol ester, as well as by stress stimuli such as Sorbitol, H2O2, and UV irradiation. WNK1 (WNK Lysine deficient protein Kinase-1) is required for activation of ERK5 by EGF. MEK5 (MAPK/ERK Kinase-5) and MEKK2/3 (MAP/ERK Kinase Kinase-2/3) acts as upstream regulators of ERK5. The known ERK5 substrates include the MEF2 (Myocyte Enhance Factor-2) family members, MEF2A, C and D, and the ETS-like transcription factor SAP1A (Signaling lymphocytic Activation molecule associated Protein-1A) (Ref.7 & 8).
The second most widely studied MAPK cascade is the JNK/SAPK (Stress Activated Protein Kinase). The JNKs/ SAPKs are encoded by at least three genes: SAPK-Alpha/JNK2, SAPK-Beta/JNK3, and SAPK-Gamma/JNK1. This cascade is activated following exposure to UV radiation, Heat shock, or Inflammatory Cytokines. Directly upstream of JNK, at the MAPKK level, there are two dual specificity kinases that phosphorylate and activate JNK at Serine and threonine residues. These kinases are MKK4 (MAPK Kinase-4), and MKK7 (MAPK Kinase-4). These proteins are activated, in turn, by the upstream MAP3K: MEKKs (MAPK/ERK Kinase Kinases), MLK2/3 (Mixed Lineage Kinase-2/3), TAK1 (TGF-Beta-Activated Kinase-1), TPL2 (Tumor Progression Locus-2), ZPK (Zipper Protein Kinase), and ASK1 (Apoptosis Signal-regulating Kinase-1). Some other MAP3Ks have also been identified, whose functions are not known. These included MAP3K6, MLK1 (Mixed Lineage Kinase-1) and LZK (Leucine Zipper-bearing Kinase). The Rho family GTPases, CDC42 and Rac initiate a cascade leading to JNK/SAPK, presumably by binding and activating the protein kinase PAK, a kinase that phosphorylates and promotes activation of MEKK1. CDC42 can also be activated by GPCR. Stimulation of GPCRs coupled to the GN-AlphaS subunit of trimeric G-proteins, induces production of cAMP and activation of PKA. Activation of PKA enhances the activity of CDC42 and thus plays an important role in activation of JNKs. The activation of JNK by Cytokine receptors appears to be mediated by the TRAF (TNF Receptor-Associated Factor) group of Adaptor proteins. Activation of the TNFR (Tumor necrosis Factor Receptor) leads to recruitment of TRAF2 (TNF Receptor-Associated Factor-2), which is required for JNK activation. This Adaptor protein (TRADD (Tumor Necrosis Factor Receptor-1-Associated Death Domain Protein), RIP (Receptor-Interacting Protein), Daxx) has been reported to bind MEKK1 and ASK1. The activated JNK/SAPKs translocate to the nucleus where they phosphorylate transcription factors such as c-Jun, c-Fos, DPC4 (Deleted in Pancreatic Carcinoma 4), p53, ATF2 (Activating Transcription Factor-2), NFAT4 (Nuclear Factor of Activated T-Cell-4), NFAT1 (Nuclear Factor of Activated T-Cell-1), STAT1 (Signal Transducers and Activators of Transcription-1), HSF1, SHC and Bcl2 (B-Cell CLL/Lymphoma-2). JNK-regulated transcription factors help to regulate gene expression in response to a variety of cellular stimuli, including stress events, Growth Factors and Cytokines. Activation of the JNK signaling cascade generally results in Apoptosis, although it has also been shown to promote cell survival under certain conditions and has important roles in determining cell fate during metazoan development as well as involvement in tumorigenesis and inflammation (Ref.9, 10 & 11).
The p38 kinase is most well-characterized member of the MAP kinase family. It shares about 50% homology with the ERKs. Four p38 MAPKs have been cloned so far in higher eukaryotes: p38-Alpha/XMpk2/CSBP, p38-Beta/p38-Beta22, p38-Gamma/SAPK3/ERK6, and p38-Delta/SAPK4. The mammalian p38 MAPK families are activated by cellular stress including UV irradiation, Heat shock, High osmotic stress, Lipopolysaccharide, Protein synthesis inhibitors, Proinflammatory Cytokines (such as IL-1 (Interleukin-1) and TNF-Alpha (Tumor Necrosis Factor-Alpha)) and certain Mitogens. The upstream MAPK cascade in p38 activation includes MAPKKKs such as ASK1, MEKK1, MEKK 4, MLK2 and 3, DLK (Dual Leucine Zipper Kinase), TPL2 (Tumor Progression Locus-2), TAK1 and TAO1/TAO2, which phosphorylate and activate MKK3 and MKK6, which in turn phosphorylate and activate p38. Proinflammatory cytokines such as IL and TNF are the main stimulator of p38. IL-1 signaling is known to involve PI3K, p38MAPK and ERK. After IL-1 is bound to its receptor IL-1R (IL-1 Receptor), a complex is formed between the Type-1 Receptor and the receptor accessory protein. The cytosolic proteins MyD88 (Myeloid Differentiation primary response gene-88) and TollIP (Toll-Interacting Protein) are recruited to this complex, where they function as adaptors, recruiting IRAK1 (IL-1 Receptor-Associated Kinase-1) in turn. IRAK1, a serine-threonine kinase, activates and recruits TRAF6 (TNF Receptor-Associated Factors-6) to the IL-1 receptor complex. Eventually, phosphorylated IRAK is ubiquitinated and degraded. TRAF6 signals through the TAB1 (TAK1 Binding Protein-1)/TAK1 (TGF-Beta-Activating Kinase-1) kinases to activate MKKs, which further activates p38MAPK (Ref.12). TNF also stimulate p38 signaling. Binding of TNFR1 to TNF-Alpha results in conformational changes in the receptor’s intracellular domain, resulting in rapid recruitment of several cytoplasmic death domain–containing adapter proteins via homophilic interaction with the death domain of the receptor. The first adaptor recruited to the clustered receptor is the TNFR–associated protein with death domain, which functions as a docking protein for several signaling molecules, such as FADD (Fas-Associated protein with Death Domain), TRADD, Daxx, TRAF2 and RIP. RIP associates with TRAF2 to generate MEKK4 and ASK1. Both MEKK4 and ASK1 activates p38MAPKs by activating MKK3 and MKK6. Besides, p38 can also be activated by GPCRs and numerous physical and chemical stresses, including hormones, UV irradiation, ischemia, osmotic shock and heat shock. G-proteins activate p38 via PKA or PKC, whereas stress activates p38 via Rac and CDC42. Following its activation, p38 translocates to the nucleus and phosphoryates ATF2. Another known target of p38 is MAPKAPK2 (MAPK-Activated Protein Kinase-2) that is involved in the phosphorylation and activation of heat-shock proteins. Other transcription factors affected by the p38 family include STAT1 (Signal Transducers and Activators of Transcription-1), Max/Myc complexes, Elk1 and CREB through the activation of MSK1 (Mitogen- and Stress-Activated Kinase-1). The p38 subfamily is also involved in affecting Cell Motility, Transcription and Chromatin Remodeling. Other substrates of the p38 signaling pathway include CHOP (C/EBP-Homologous Protein) for regulation of gene expression, as well as MNK1 (MAPK-Interacting Kinase-1). p38 MAPK is a crucial mediator in the NF-kappaB-dependent gene activation induced by TNF (Ref.13, 14 & 15).
The mammalian MAPK signaling system employ scaffold proteins, in part, to organize the MAPK signaling components into functional MAPK modules, thereby enabling the efficient activation of specific MAPK pathways. The ERK scaffold protein KSR (Kinase Suppressor of Ras) binds ERK, its direct activator MEK and Raf. A second targeting protein, p14, targets ERK2 to an endosomal location through its interaction with MP1 (MAPKK1-Interacting Protein-1), an adaptor protein that binds MEK and ERK. In addition, MEKK1 (MAP/ERK Kinase Kinase-1) can serve both as a scaffold and as MAPKKK, interacting specifically with MAPKK and MAPK. Multidomain protein Posh (Plenty of SH3s) acts as a scaffold for the JNK pathway. Posh binds MLKs both in vivo and in vitro, and complexes with MKKs 4 and 7 and with JNKs. The JNK MAPK modules are also regulated by a JIP1 (JNK Interacting Protein-1), JIP2 (JNK Interacting Protein-2), JIP3 (JNK Interacting Protein-3), JIP4 (JNK Interacting Protein-4), Beta-Arrestin-2, Filamin and CrkII. There is increasing evidence that the three well-characterized members of the MAPK family, ERK1/2, JNK/SAPK and p38 play an important role in regulation of proliferation in mammalian cells by sharing substrate and cross-cascade interaction. MAPK pathways are involved in many pathological conditions, including cancer and other diseases. Therefore, a better understanding of the relationship between MAP kinase signal transduction system and the regulation of cell proliferation is essential for the rational design of novel pharmacotherapeutic approaches (Ref.16 & 17).
References
- 1
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation.
- 2
- Panka DJ, Atkins MB, Mier JW. Targeting the mitogen-activated protein kinase pathway in the treatment of malignant melanoma.
- 3
- Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death.
- 4
- Osmond RI, Sheehan A, Borowicz R, Barnett E, Harvey G, Turner C, Brown A, Crouch MF, Dyer AR. GPCR screening via ERK 1/2: a novel platform for screening G protein-coupled receptors.
- 5
- Ginnan R, Guikema BJ, Singer HA, Jourd
- 6
- Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase.
- 7
- Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway.
- 8
- Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK5 binds to and phosphorylates p90 RSK.
- 9
- Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG. Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the MAP kinase JNK.
- 10
- Yamauchi J, Miyamoto Y, Kokubu H, Nishii H, Okamoto M, Sugawara Y, Hirasawa A, Tsujimoto G, Itoh H. Endothelin suppresses cell migration via the JNK signaling pathway in a manner dependent upon Src kinase, Rac1, and Cdc42.
 关于我们
关于我们