IP3_Pathway
发布时间:2019-12-11 09:14 来源:SABiosciences
- 通路
- 概述
Review
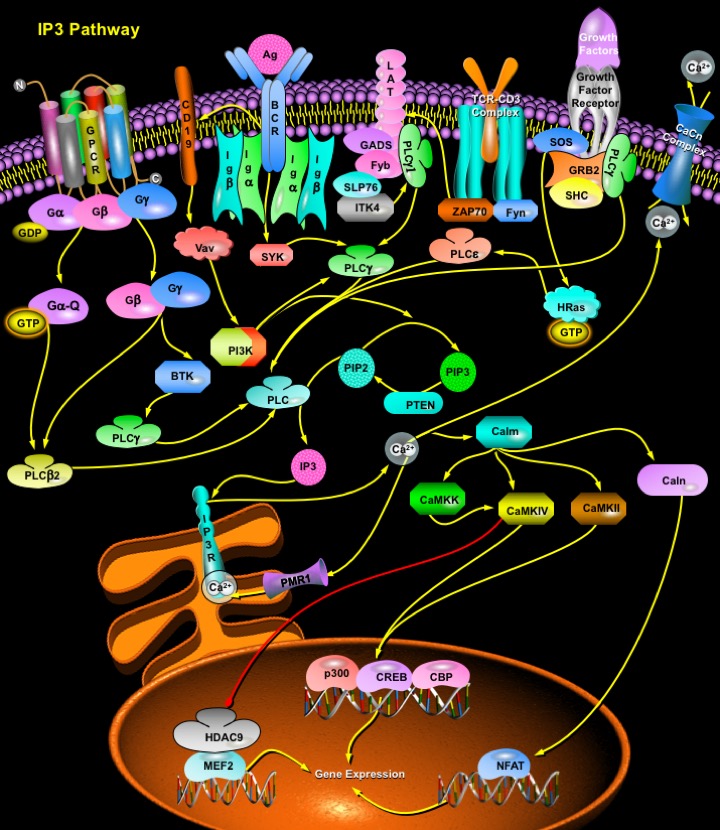
IP3 (Inositol 1,4,5-triphosphate), also known as a second messenger, is a molecule that functions to transfer a chemical signal received by the cell, such as from a hormone, neurotransmitters, growth factors and hypertrophic stimuli such as AngII (Angiotensin-II), Beta-adrenergic receptor agonists, and ET1 (Endothelin-1) to various signaling networks within the cell. IP3 is known to play a crucial role in initiating and propagating these messages; however, the precise mechanism of how IP3 relates to the next element in its signaling pathway, the calcium wave, remains highly controversial. The receptors for IP3, IP3R (IP3 Receptor) constitute a family of Ca2+ channels responsible for the mobilization of intracellular Ca2+ stores. Three different receptor types have been molecularly cloned, and their genes have been classified into a family. IP3Rs are tetramers that act as ligand-gated channels facilitating Ca2+ release from internal stores/endoplasmic reticulum. The primary structure of the IP3R contains 3 domains: an Inositol triphosphate binding domain near the N terminus, a coupling domain in the middle of the molecule, and a transmembrane spanning domain near the C terminus (Ref.1).
Two essential signaling pathways involve the intracellular generation of Inositol Phosphates. The first signaling pathway is initiated by PLC (Phospholipase-C). PLC are soluble proteins that are partly cytosolic and partly associated with membrane. Based on their functional and structural characteristics, they have been grouped into four classes: PLC-Delta, -Beta, -Gamma and -Epsilon. Hormones and neurotransmitters bind to GPCR (G-Protein Coupled Receptors) and both the heterotrimeric G-AlphaQ/11 and G-Beta Gamma subunits regulate the function of PLC-Beta. Other members of the PLC family are activated by growth factors that activate RTK (Receptor Tyrosine Kinases). Most growth factors that signal through RTKs stimulate Ras by recruiting the GEF (Guanine-Nucleotide Exchange Factor) SOS (Son of Sevenless) to the membrane. SOS exists in a complex with the adapter protein GRB2 (Growth Factor Receptor-Bound Protein-2) and SHC. Upon receptor activation, the GRB2/SOS complex is translocated to the membrane by binding of GRB2 to tyrosyl-phosphorylated residues in RTKs or additional adapter proteins and activate H-Ras. Ras is an activator of PLC-Epsilon. PLC-Gamma, on the other hand, can be activated by antigen-stimulated activation of non-receptor tyrosine kinases such as Src via binding to TCRs (T-Cell Receptors) and BCRs (B-Cell Receptors). Ligation of the TCR triggers the activation of Lck and Fyn (Fyn Oncogene Related to Src, FGR, YES) by unknown mechanisms. Either or both of these Src family PTKs then phosphorylates tyrosine residues within ITAM sequences located in TCR and CD3 chains. Two phosphorylated tyrosine residues with this motif serve as binding sites for the tandem SH2 domains of ZAP70 (Zeta-Chain-Associated Protein Kinase). Lck or Fyn then phosphorylates the bound ZAP70, resulting in its activation. Together with Lck and Fyn, activated ZAP70 phosphorylates various downstream substrates, including membrane-bound LAT (Linker for Activation of T-Cells) and SLP76 (SH2 Domain-Containing Leukocyte Protein-76). The interaction of the N-SH2 domain of PLC-Gamma1 with a phosphorylated tyrosine residue of LAT serves to position the unphosphorylated enzyme close to activated ZAP70 and Lck or Fyn, resulting in the phosphorylation and activation of PLC-Gamma1 and in its localization in the vicinity of its substrate. Phosphorylated LAT also associates with GADS, which might in turn associate with ITK (IL-2 inducible T-cell kinase)-bound SLP76; the close proximity of ITK and PLC-Gamma1 may result in the phosphorylation by ITK of PLC-Gamma1. BCR engagement triggers the activation of Lyn by an unknown mechanism. Activated Lyn phosphorylates tyrosine residues within ITAM sequences located in the Ig-Alpha and Ig-Beta chains. The two phosphorylated tyrosines within this motif serve as binding sites for the tandem SH2 domains of SYK (Spleen Tyrosine Kinase), and the ITAM-bound SYK is phosphorylated (activated) by Lyn. Activated SYK phosphorylates BTK (Bruton Agammaglobulinemia Tyrosine Kinase) and activated BTK phosphorylates PLC-Gamma2. Activated SYK can also directly phosphorylate PLC-Gamma2 (Ref.2, 3 & 4).
Activation of mammalian Phosphoinositide-specific PLC results in the hydrolysis of PIP2 (Phosphatidylinositol-4,5-Bisphosphate) to release the second messengers DAG (1,2-Diacylglycerol) and IP3. DAG is the physiological activator of PKC (Protein Kinase-C), which in turn activates the ERK1/2 (Extracellular Signal Regulated Kinase-1/2) signaling pathway resulting in transcription factor activation and cell survival (Ref.5). IP3, which accumulates rapidly and transiently, binds to intracellular receptors, IP3R in the intracellular stores like ER (Endoplasmic Reticulum) and activates Ca2+ release from the ER lumen to the cytoplasm, generating complex cytoplasmic Ca2+ concentration signals including temporal oscillations and propagating waves. Ca2+ release from the intracellular stores is mediated by RyR (Ryanodine Receptor), PMR1 and IP3R channels. RyR are activated by a rise in intracellular CICR (Ca2+ Induced Ca2+ Release). In addition there are RyR-like channels activated by cADPR (cyclic ADP-ribose), Sphingosine and a distinct Ca2+-release pathway activated by NAADP (Nicotinic Acid Adenine Dinucleotide Phosphate). Within Ca2+-storing organelles, Ca2+ ions are bound to specialized Ca2+-buffering proteins. These include CS (Calsequestrins), CR (Calreticulins) and CN (Calnexins). In the cytosol, there are mobile Ca2+ buffers; the CB (Calbindins), PV (Paravalbumin), Calm (Calmodulin) and S100 protein families that blunt Ca2+ spikes and assist in redistribution of Ca2+ ions. The Ca2+ release from internal stores occurs through channels formed by IP3Rs, and entry is mediated by a set of functionally heterogeneous but ubiquitous channels that are activated by the store depletion event. Ca2+ release activates Calm (Calmodulin) which further activates Caln (Calcineurin), CamKKs and CamKs (CamK4 and CamK2). Caln facilitates NFAT (Nuclear Factor of Activated T-Cells) translocation to the nucleus, a process that is quite essential for axonal growth. CamK4 and CamK2 phosphorylates CBP (CREB Binding Protein) and Histone Deacetylases, HDAC4, HDAC5 and HDAC7 , which mediates some nuclear Ca2+ signals. HDAC export allows MEF2 (Myocyte Enhancing Factor-2) to activate transcription by recruiting other Ca2+-sensitive transcriptional factors such as NFAT (Nuclear Factor of Activated T-Cells) and transcriptional coactivators such as p300. CREB (cAMP Response Element-Binding Protein) can be phosphorylated by CamK4at a number of sites other than Ser133, including Ser129, Ser142 and Ser143. Phosphorylation of Ser142 and dephosphorylation of Ser133 residue by CamK represses CREB activity (Ref.6 & 7).
The second signaling pathway involving IP3 generation is initiated by PI3K (Phosphoinositide 3-Kinase), an enzyme that phosphorylates inositol lipids generating two signaling molecules, PIP2 (Phosphatidylinositol 3,4-Bisphosphate) and PIP3 (Phosphatidylinositol 3,4,5-Trisphosphate). PIP2 and PIP3 function as activators of protein kinases and may also regulate G proteins. PI3K is activated in B-Cells by CD19 (CD19 Antigen). CD19 is a co-receptor complex in B-Cell, which is activated by BCR. In B-Cells, PI3K functions upstream of PLC-Gamma2 but activation of PLC-Gamma2 can also occur independently of PI3K. IP3, generated from PIP2 has a vital role in the control of cellular and physiological processes as diverse as cell division, cell proliferation, apoptosis, fertilization, development, behaviour, memory and learning (Ref.8).
References
- 1
- Szlufcik K, Missiaen L, Parys JB, Callewaert G, De Smedt H. Uncoupled IP3 receptor can function as a Ca2+-leak channel: cell biological and pathological consequences.
- 2
- Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases.
- 3
- Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks AR. Regulation of the type 1 inositol 1,4,5-trisphosphate receptor by phosphorylation at tyrosine 353.
- 4
- Zhu DM, Tibbles HE, Vassilev AO, Uckun FM. SYK and LYN mediate B-cell receptor-independent calcium-induced apoptosis in DT-40 lymphoma B-cells.
- 5
- Hisatsune C, Nakamura K, Kuroda Y, Nakamura T, Mikoshiba K. Amplification of Ca2+ signaling by diacylglycerol-mediated inositol 1,4,5-trisphosphate production.
- 6
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism.
- 7
- Yamazaki H, Michikawa T, Mikoshiba K. IP3 receptor and calcium signaling.
- 8
- Dai X, Chen Y, Schuman J, Hua Z, Adamson JW, Wen R, Wang D. Distinct Roles of Phosphoinositide-3 Kinase and Phospholipase C{gamma}2 in B-Cell Receptor-Mediated Signal Transduction.
 关于我们
关于我们
