Inhibition_of_Angiogenesis_by_TSP1
发布时间:2019-12-10 16:18 来源:SABiosciences
- 通路
- 概述
Review
Cancer is a multistep process that includes deregulation of cell cycle, transformation, invasion of stroma, angiogenesis and metastasis. Angiogenesis is an essential component for tumor development regulated by both proangiogenic and antiangiogenic factors (Ref.1). It is a multi-step process that includes endothelial cell proliferation, migration, basement membrane degradation, and new lumen organization. Naturally occurring inhibitors of angiogenesis i.e., antiangiogenic factors are found in mammalian tissues, where they help maintain the quiescence of the normal vasculature. Thus, angiogenic inhibitors have been considered as potential anticancer drugs. TSP1 (Thrombospondin-1) is a naturally occurring inhibitor of angiogenesis that limits vessel density in normal tissues and curtails tumor growth (Ref.2).
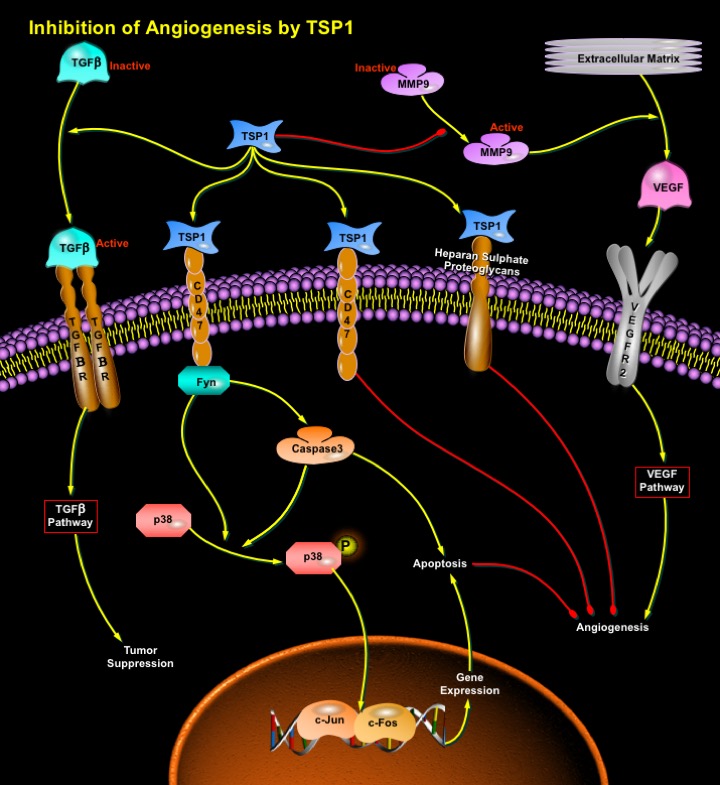
Thrombospondins are a family of extracellular matrix proteins that have diverse effects on cell adhesion, motility, proliferation, and survival. Two members of this family, TSP1 and TSP2 act as inhibitors of angiogenesis (Ref.3). TSP1 is a large, multifunctional glycoprotein that influences cellular phenotype and the structure of the extracellular matrix. These effects are important components of the tissue remodeling that is associated with angiogenesis and neoplasia. TSP1 inhibits angiogenesis through direct effects on endothelial cell migration and survival and through indirect effects on growth factor mobilization. The TSP1 that is produced by stromal fibroblasts, endothelial cells and immune cells, suppresses tumor progression. In the tumor microenvironment, TSP1 acts to suppress tumor cell growth through activation of TGF-Beta (Transforming Growth Factor-Beta) in those tumor cells that are responsive to TGF-Beta (Ref.4).
TSP1 binds to a wide variety of integrin and non-integrin cell surface receptors. The binding sites for these receptors on TSP1 are dispersed throughout the molecule, with most domains binding multiple receptors. In some cases, TSP1 can also bind to multiple receptors concurrently (Ref.5). The inhibitory effects of TSP1 are mediated by the binding of TSP1 to an array of cell-surface receptors, including CD36, CD47, and heparan sulfate proteoglycan receptors (Ref.3). The induction of apoptosis by TSP1 in endothelial cells requires the sequential activation of CD36, the Src-family tyrosine kinase: p59Fyn, Caspase3 like proteases and p38 MAPK (Mitogen-Activated Protein Kinases). This is accomplished, perhaps through the activation of Activator complex-1 (c-Jun and c-Fos) and the subsequent activation of genes that lead to apoptosis. Induction of apoptosis is regarded an essential substep for the inhibition of angiogenesis by TSP1. Caspase-3-like protease is required for the activation of p38MAPK (Ref.2). TSP1 induces a rapid and transient activation of JNK (c-Jun N-terminal Kinases). JNK activation by TSP1 also requires engagement of CD36 (Ref.6).
In tumors, TSP1 that is secreted by stromal cells and some tumor cells directly inhibits endothelial cell migration and survival, that stimulates endothelial cell apoptosis. These effects result in the down-regulation of angiogenesis and the inhibition of tumor growth. In addition to activating apoptotic pathways, TSP1 is also involved in the downregulation of survival pathways (Ref.4). The indirect effects of TSP1 on tumor growth result from its ability to activate TGF-Beta and inhibit exracellular MMPs (Matrix Metalloproteinases). The generation of active TGF-Beta by TSP1 in the stroma suppresses the growth of tumor cells that remain responsive to TGF-Beta and the inhibition of MMPs, specifically MMP9, inhibits the mobilization of VEGF (Vascular Endothelial Growth Factor). VEGF is a potent agonist of angiogenesis that activates both endothelial cell proliferation and migration. The inhibition of MMP9 activation by TSP1 decreases the amount of VEGF that is liberated from the extracellular matrix. The level of active MMP9 is directly proportional to the level of VEGF that is bound to VEGFR2 on endothelial cells within these tumors. Thus, it indicates that TSP1 inhibits angiogenesis by blocking activation of MMP9 and, consequently, the release of VEGF that is sequestered in the extracellular matrix. Thus, TSP1 inhibits angiogenesis directly by inducing endothelial cell apoptotic pathways while indirect effects on tumor cells involve activation of TGF-Beta and inhibition VEGF-activated survival pathways (Ref.1).
Factors that inhibit angiogenesis might act as cancer therapeutics. TSP1, being an antiangiogenic factor, inhibits angiogenesis and slows tumor growth. TSP1 expression is positively regulated by the PTEN (Phosphatase and Tensin Homolog), SMAD, and p53 tumor suppressor proteins. It can also be down-regulated by activation of oncoproteins such as ras, src, myc, and c-jun as well as by a novel metastasis-associated gene product, MTS1 (Multiple Tumor Suppressor-1). Many cells express TSP1, and low levels of TSP1 expression have been associated with increased recurrence rates and decreased overall survival in several human cancers, suggesting that the loss of TSP1 is critical for tumor development (Ref.1). Since the endogenous levels of TSP1 are not capable of producing the maximum level of inhibition, therapeutic approaches that are designed to increase TSP1 levels should be effective for the inhibition of tumor growth. To date, little attention has been given to the effects of TSP1 on immune cells that are recruited to the tumor microenvironment. In addition, the effect of TSP1 on gene expression within tumor and stromal cells is yet to be stressed upon. The relative importance of the various mechanisms for inhibition of tumor growth by TSP1 will probably vary in the different types of tumors. A complete understanding of the role of TSP1 in tumor progression will permit the design of therapies that specifically target these mechanisms (Ref.2).
References
- 1
- Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation.
- 2
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1.
- 3
- Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1.
- 4
- Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth.
- 5
- Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1.
- 6
- Jimenez B, Volpert OV, Reiher F, Chang L, Munoz A, Karin M, Bouck N. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1.
 关于我们
关于我们
