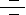IL-2_Pathway
发布时间:2019-12-10 16:04 来源:SABiosciences
- 通路
- 概述
Review
IL-2 (Interleukin-2) is a T-Cell-derived cytokine important in the regulation of growth and differentiation of T-Cells, B-Cells, natural killer cells, glioma cells, and cells of the monocyte lineage after specifically interacting with its receptors. Human IL-2 is a 133-amino acid polypeptide with a molecular mass of 15-18 kDa. IL-2 signaling is mediated by a multichain receptor complex consisting of an alpha (CD25), beta (CD122), and gamma (CD132) chain. The IL-2R (IL-2 Receptor) alpha subunit primarily increases the affinity of ligand binding and is not known to contain a signaling domain, whereas the beta and gamma subunits participate in both ligand binding and signal transduction (Ref.1). The IL-2R signaling system proceeds through at least three different pathways, which mediate the flow of mitogenic and survival-promoting signals. One of the pathways proceeds through protein tyrosine kinase activity, Ras and the MAPK (Mitogen-Activated Protein Kinase) cascade, leading to expression of the protooncogenes c-Fos, c-Jun, and Elk1. The Syk, that is responsible for c-Myc gene induction, initiates the second pathway. The final pathway results in BCL2 (B-Cell Leukemia-2) expression, and progression through a Rho, PI3K (Phosphoinositide-3 Kinase) and Akt/PKB (Protein Kinase-B) mediated signaling pathway. This last pathway is also involved in IL-2-promoted regulation of actin cytoskeleton organization (Ref.2).
IL-2R signaling activates PI3K which catalyses phosphorylation of inositol phosphates. These act as second messengers and recruit molecules such as Akt kinase to the cell membrane. Akt kinase is further activated by phosphorylation and subsequently positively or negatively regulates the activity of downstream targets like PKB. Proapoptotic proteins which can be phosphorylated and inhibited by PKB include BAD (BCL2 Antagonist of Cell Death), human Caspase9, and the forkhead family of transcription factors. PKB can also cause stimulation of NF-KappaB (Nuclear Factor-Kappa B) activity by upregulating I-KappaB degradation via phosphorylation of IKKs (I-KappaB Kinases) and by affecting NF-KappaB itself, thereby allowing the transcription of genes involved in promoting survival, such as the BCL2 homologue bfl-1. In addition to the forkhead and NF-KappaB families, E2F-mediated transcription can also be activated by the hyperphosphorylation and subsequent inactivation of Rb (Retinoblastoma Protein) in response to signals from PI3K and its downstream effectors, PKB and p70S6K. The transcription factors activated by PI3K and PKB are of great interest in the IL-2 response, as they regulate the genes responsible for determining whether activated T-Cells survive, proliferate, or die (Ref.3). Recently, it has been shown that the SHP2 (SH2 Containing Phosphatase), becomes phosphorylated in response to IL-2 stimulation, associates with PI3K and GRB2 (Growth Factor Receptor Bound Protein 2), and can exert a positive regulatory role in IL-2 signaling.
IL-2 also activates Lck (Lymphocyte-Specific Protein Tyrosine Kinase), which is involved in T-cell receptor signaling. Signaling from the T-cell receptor does activate PLC-Gamma, but this requires ligand binding. The protein tyrosine kinases JAK1 and JAK3 (Janus Kinases-1 and -3), which are associated with the IL-2R beta and gamma subunits, respectively, are also activated after binding of IL-2 to its receptor. Phosphorylation of the cytoplasmic domains of the betaƒn- and gammaƒn-subunits of the IL-2R provides docking sites for the JAK1/3, which, after autophosphorylation, in turn provide docking sites for and phosphorylates STAT3 (Signal Transducer and Activator of Transcription-3) and STAT5. Phosphorylation induces dimerisation and nuclear translocation of STAT3 and STAT5 complexes, where they promote specific target gene transcription. IL-2 also stimulates ERKs (Extracellular Signal Regulated Protein Kinases) and/or p38 in Mitogen-activated T lymphocytes. Several transcription factors including NFAT (Nuclear Factor of Activated T-Cells), Activating Protein1, and NF-KappaB have been identified as major regulators of IL-2. NFAT is activated mainly by a Ca2+ (Calcium)-dependent protein phosphatase; calcinuerin. The increase of intracellular Ca2+ activates this phosphatase and induces nuclear translocation of NFAT. Activating Protein-1, which consists of two transcription factors, Fos and Jun, is another key component for activation of the IL-2 promoter. Activating Protein-1 binding sites are found in juxtaposition to NFAT and NF-KappaB binding sites, and it has been shown that NFAT and Activating Protein-1 function in a cooperative manner. Binding sites for NF-KappaB, a group of transcription factors involved in the regulation of many genes are found in the IL-2 promoter region, and members of the NF-KappaB family, RelA and c-Rel, are activated by TCR (T-Cell Receptor) stimulation (Ref.4). Recently, it has been shown that the SH2-containing phosphatase, SHP-2, becomes phosphorylated in response to IL-2 stimulation, associates with PI3K and GRB2, and can exert a positive regulatory role in IL-2 signaling. IL-2 is associated with cell-mediated immune response. Recombinant IL-2 (aldesleukin, Proleukin) is used for cancer therapy and continues to be studied as an immunomodulatory treatment for Kaposi's sarcoma and HIV disease. Side effects include flu-like symptoms (fever, chills), decreased blood pressure, and anorexia (Ref.5).
References
- 1
- Smith KA. Interleukin-2: inception, impact, and implications.
- 2
- Gómez J, García-Domingo D, Martínez-A C, Rebollo A. Role of NF-kappaB in the control of apoptotic and proliferative responses in IL-2-responsive T cells.
- 3
- Lindemann MJ, Benczik M, Gaffen SL. Anti-apoptotic signaling by the interleukin-2 receptor reveals a function for cytoplasmic tyrosine residues within the common gamma (gamma c) receptor subunit.
- 4
- Iwashima M, Takamatsu M, Yamagishi H, Hatanaka Y, Huang YY, McGinty C, Yamasaki S, Koike T. Genetic evidence for Shc requirement in TCR-induced c-Rel nuclear translocation and IL-2 expression.
- 5
- Cacalano NA, Johnston JA. Interleukin-2 signaling and inherited immunodeficiency.
 关于我们
关于我们