Human_Embryonic_Stem_Cell_Pluripotency
发布时间:2019-12-10 15:52 来源:SABiosciences
- 通路
- 概述
Review
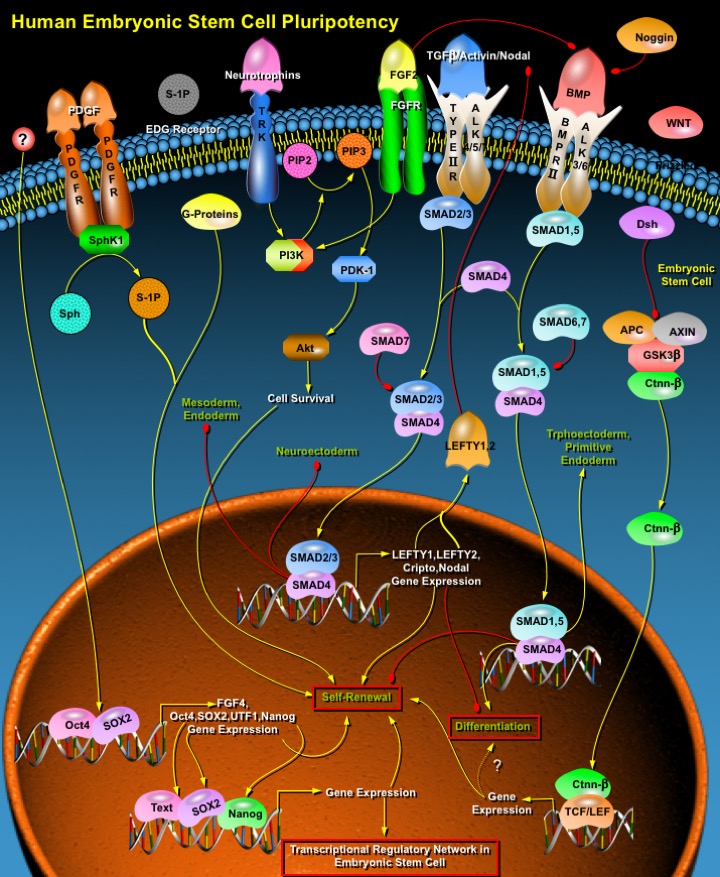
ESCs (Embryonic Stem Cells) are Pluripotent cells capable of differentiating into any cell type of the body. Only three species of Mammals have yielded long-term cultures of self-renewing ESCs- Mice, Monkeys, and Humans. Human ESCs are derived from Blastocysts, multicellular structures originating from four cleavages of fertilized oocytes. Isolated from the ICM (Inner Cell Mass) of Blastocysts, the ESCs retain properties of self-renewal and the potential to be committed and to differentiate toward most cell lineages. They are able to spontaneously give rise to different progenies of the three embryonic layers, namely, the Ectoderm, the Mesoderm and the Endoderm. The Pluripotency of ESCs has attracted great attention for their potential use in tissue and cell therapy. However, the molecular and developmental mechanisms controlling Pluripotency and Differentiation of ESCs are largely unknown. Human ESC Pluripotency is regulated by a combination of Extrinsic and Intrinsic factors. Unlike Mouse, Extrinsic factor LIF (Leukemia Inhibitory Factor) is not sufficient to maintain Human ESC and BMPs (Bone Morphogenic Proteins) cause rapid differentiation. Instead, FGF (Fibroblast Growth Factor) signaling and a balance between TGF-Beta (Transforming Growth Factor-Beta) /Activin and BMP signaling are central to the self-renewal of Human ESCs. Intrinsic factors regulating Pluripotency in Human ESCs include a battery of transcription factors including Oct4 (Octamer Binding Transcription Factor-4), SOX2 (SRY (Sex Determining Region-Y) Box-2) and Nanog (Ref.1 & 2).
TGF-Beta superfamily of ligands, which contains about 40 potential ligands in the Human genome, play a major role maintaining the Self-renewing Human ESCs. They signals through two main branches: the SMAD1/5 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-1/5) branch, which transduces on behalf of BMP and GDF (Growth Differentiation Factor) ligands via the Type I receptors ALK1 (Activin Receptor-Like Kinase-1), ALK2 (Activin Receptor-Like Kinase-2), ALK3 (Activin Receptor-Like Kinase-3) and ALK6 (Activin Receptor-Like Kinase-6); and the TGF-Beta/Activin/Nodal branch, which involves the activation of SMAD2/3 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-2/3) via ALK4 (Activin Receptor-Like Kinase-4), and ALK5 (Activin Receptor-Like Kinase-5) and ALK7 (Activin Receptor-Like Kinase-7). There are also two inhibitory SMADs – SMAD6 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-6), which selectively inhibits SMAD1/5; and SMAD7 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-7), which inhibits both branches of TGF-Beta signaling, that provide a repressive input on the pathway. Upon activation by phosphorylation and association with a common SMAD4 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-4), the receptor-activated SMADs translocate to the nucleus and, in concert with other transcription factors, regulate gene expression. Activation of the TGF-Beta/Activin/Nodal branch through SMAD2/3 is associated with Pluripotency and is required for the maintenance of the undifferentiated state in Human ESCs. SMAD2/3 pathway is also required for positive regulation of several factors of TGF-Beta signaling. These factors include Nodal, Cripto, LEFTY1 (Left-right determination factor-1) and LEFTY2 (Left-right determination factor-2). Cripto encodes an EGF-CFC co-receptor that is essential for responsivity to Nodal, and LEFTY1 and LEFTY2 are both inhibitors of Nodal signaling. Expression of Nodal, LEFTY1 and LEFTY2 remain high in undifferentiated Human ESCs and reduce upon differentiation (Ref.3, 4 & 5).
In contrast to TGF-Beta/Activin/Nodal signaling, which promotes the maintenance of Pluripotent Human ESCs, BMP signaling is unable to support self-renewal and is associated with differentiation to Trophoblast or Extraembryonic Endoderm cells. In Human ESCs, BMP4 (Bone Morphogenic Protein-4) induces differentiation into Mesoderm and Ectoderm, whereas BMP2 (Bone Morphogenic Protein-2) promotes Extraembryonic Endoderm differentiation. Repression of BMP signaling in Human ESCs by Noggin and FGF supports long term Self-renewal. FGF2 (Fibroblast Growth Factor-2) is known as the best known factor promoting Self-renewal in Human ESCs. Exogenous FGF2 is capable of maintaining Human ESCs in the absence of serum and feeder cells. Binding of FGF to its receptor and Heparin leads to receptor autophosphorylation and activation of intracellular signaling cascades, including the Ras/ERK (Extracellular Signal-Regulated Kinase) pathway, the PLC-Gamma (Phospholipase-C-Gamma)/Ca2+ (Calcium) pathway, and the PI3K (Phosphoinositide 3-Kinase) pathway. FGF2 promote self-renewal of Human ESCs by activating the PI3K pathway. PI3K catalyzes the conversion of
PIP2 (Phosphatidylinositol (3,4)-bisphosphate) to PIP3 (Phosphatidylinositol (3,4,5)-trisphosphate). The binding of PIP3 to the PH domain anchors Akt to the Plasma membrane and allows its phosphorylation and activation by PDK-1 (Phosphoinositide-Dependent Kinase-1). Activated Akt promotes cell proliferation, survival, growth, and motility and is implicated in tumorogenicity. Survival of cell leads to Pluripotency. FGF2- dependent PI3K/Akt activation is required for the efficient expression of ECM (Extracellular Matrix) proteins. Role of PI3K in maintaining Human ESC self-renewal requires further investigation. A role for PI3K has been proposed in Human ESC survival, where it mediates the survival activity of Neurotrophins, signaling through TRK Receptors (Tyrosine Kinase Receptors) (Ref.3, 6, 7 & 8).
WNTs (Wingless-Type MMTV Integration Site Family Members) proteins are also believed to play an important role in controlling ESC maintenance. Canonical WNT signaling involves the binding of WNT ligands to the Frizzled receptors. This, in turn, activates Dsh (Dishevelled), which displaces GSK-3Beta (Glycogen Synthase Kinase-3Beta) from the APC (Adenomatous Polyposis Coli) /AXIN (Axis Inhibitor) complex, preventing Ubiquitin-mediated degradation of Ctnn-Beta (Catenin-Beta). Subsequently, Ctnn-Beta accumulates and translocates into the nucleus where it associates with TCF (T Cell Factor)/ LEF (Lymphoid Enhancer Factor) proteins to activate transcription of WNT target genes. WNT signaling is known to be involved in regulating the proliferation of Stem cells, including Intestinal and Hematopoietic cells, and the Self-renewal of Hematopoietic stem cells. Components of the WNT signaling pathway are present in Human ESCs, although levels of different receptors varied between undifferentiated and differentiated populations. WNT is believed to stimulate Human ESC proliferation as well as differentiation (Ref.3, 9 & 10).
S1P (Sphingosine-1-Phosphate), a bioactive Lysophospholipid, also supports Human ESC self-renewal. S1P signals both extracellularly, through EDG (Endothelial Differentiation Gene) receptors coupled to G-Proteins, and intracellularly by undefined mechanisms. S1P has been implicated in a diverse range of biological processes, including cell growth, differentiation, migration and apoptosis in many different cell types. Because the prevention of apoptosis is a common self-renewal mechanism, S1P potentially aids the self-renewal process in Human ESCs cells. PDGF (Platelet Derived-Growth Factor) has also been implicated in the prevention of apoptosis. PDGF promotes intracellular S1P signaling by activating SphK (Sphingosine Kinase), which in turn converts Sphingosine to S1P. The combination of Extracellular PDGF and S1P supports Human ESC self-renewal (Ref.11 & 12).
The undifferentiated state of Human ESCs is also maintained by the action of transcription factors some of which are ESC-specific and common between Human and Mouse ESCs. Major transcription factors regulating Pluripotency include Oct4, SOX2 and Nanog. The best-characterized gene of these is Oct4, which functions to maintain Pluripotency both in vivo and in vitro. Oct4 is a POU domain transcription factor that is specifically expressed in all Pluripotent cells. There is scarce knowledge concerning the upstream factors that regulate the expression of the key transcription factors. The expression of Oct3/4 is regulated by a proximal enhancer and promoter in the Epiblast and by a distal enhancer and promoter at all other stages in the Pluripotent cell lineage. Limited downstream target genes of Oct3/4 have been identified, out of which FGF4 is the most common target. SOX2, a Sry-related transcription factor, activates the transcription of target genes such as FGF4, in cooperation with Oct3/4. SOX2 expression is controlled by Oct4 and SOX2, suggesting a positive feedback mechanism that is related to the maintenance of ESC self-renewal. Two regulatory elements, SRR1 and SRR2, have been found in the area of the SOX2 gene in undifferentiated ES cells. In the presence of these two regulatory elements, a high level of gene expression is achieved through synergistic activity. The partnership between Oct3/4 and SOX2 is involved in the expression of the UTF1 (Undifferentiated embryonic cell Transcription Factor-1) gene found to encode an ESC-specific co-activator. The binding sites for both factors are located in the second intron of the UTF1 gene with no intervening spaces. Thus, Oct3/4 SOX2 partnership is indispensable in the maintenance of Pluripotency. Stellar, a gene with similar expression to Oct3/4 has recently been identified. Its expression is mainly restricted to Pluripotent Human ESCs, Premeiotic Germ cell tumors, and Testicular Germ cell tumors (Ref.13, 14 & 15).
Nanog is another member of the group of transcription factors whose functions are deemed essential for the process of Self-renewal in Human ESCs. Nanog is a NK2-family homeobox transcription factor and it acts by transcriptional activation, achieved by binding to homeobox domains in downstream target genes. Analogous to Oct3/4 and SOX2, Nanog expression is high in Human ESCs and is down regulated as cells differentiate. Transcription of Nanog is regulated by the binding of Oct3/4 and SOX2 to the Nanog promoter. Oct3/4, SOX2, and Nanog co-occupy the promoters of a large range of genes, many of which encode developmentally important transcription factors. Oct3/4, SOX2, and Nanog together occupy a minimum of 353 of Human ESC genes to maintain Pluripotency. Thus, Human ESCs exhibit a number of signaling pathways involved in Self-renewal and Pluripotency that are interdependent and display a range of cross-talk mechanisms. Deciphering these pathways holds promise for generating improved methodologies for maintenance and proliferation of Pluripotent Human ESCs in vitro. Together, the understanding of the exogenous and endogenous factors determining cell fate will facilitate the use of these cells in cell-based therapies and will allow understanding of early developmental processes (Ref.2, 16, 17 & 18).
References
- 1
- Biswas A, Hutchins R. Embryonic stem cells.
- 2
- Darr H, Benvenisty N. Human embryonic stem cells: the battle between self-renewal and differentiation.
- 3
- Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells.
- 4
- Besser D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3.
- 5
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells.
- 6
- Conley BJ, Ellis S, Gulluyan L, Mollard R. BMPs regulate differentiation of a putative visceral endoderm layer within human embryonic stem-cell-derived embryoid bodies.
- 7
- Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, Meng S, Feng J, Miao C, Ding M, Li D, Deng H. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers.
- 8
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells.
- 9
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency.
- 10
- Davidson KC, Jamshidi P, Daly R, Hearn MT, Pera MF, Dottori M. Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells.
 关于我们
关于我们
