HGF
发布时间:2019-12-10 15:40 来源:SABiosciences
- 通路
- 概述
Review
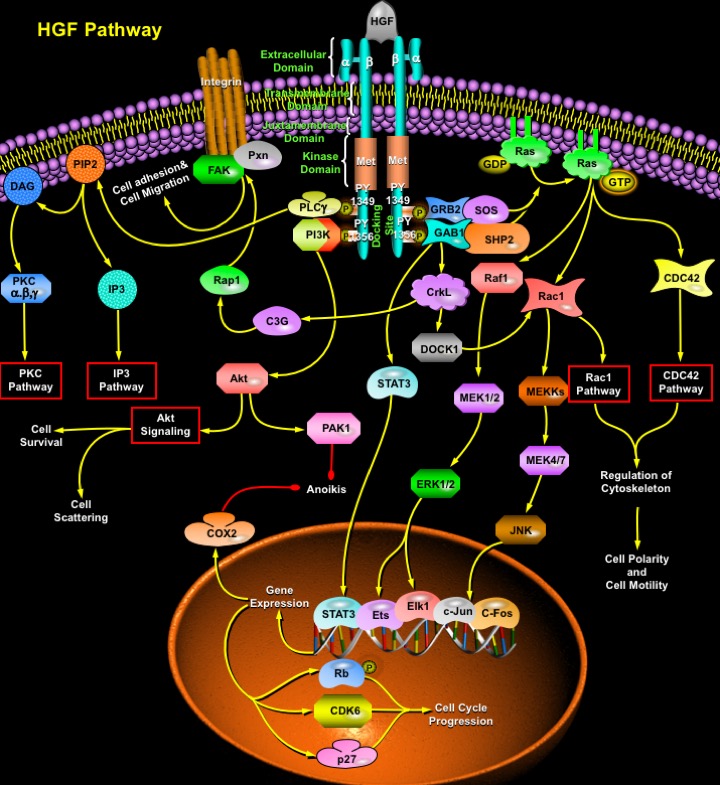
HGF (Hepatocyte Growth Factor)/SF (Scatter Factor) is a mesenchymal- or stromal-derived multipotent heparan sulfate-binding and dermatan sulfate-binding pleiotropic polypeptide that mediates epithelial-mesenchymal interactions with mitogenic, motogenic and morphogenic activities towards many normal and neoplastic epithelial cells. Initially identified as a potent hepatotrophic factor responsible for vigorous regeneration of the liver, it has now become a well characterized multipotent cytokine with biological functions that reach far beyond the original identifications, operating in virtually every tissue of the body, the cellular targets being the hepatocytes and other epithelial cells, melanocytes, endothelial and haematopoietic cells (Ref.1). During embryogenesis, HGF stimulates cell proliferation (e.g. placental cytotrophoblasts), differentiation, motility and invasiveness, induces angiogenesis, is involved in haematopoiesis, chondrogenesis, and supports organogenesis and morphogenesis of various tissues and organs, including the liver, kidney, lung, mammary gland, tooth, skeletal system, etc. In adult tissues, HGF elicits a potent organotrophic function, which supports regeneration of organs including the liver, kidneys, and lungs. HGF is a natural ligand for the c-Met proto-oncogene product of receptor tyrosine kinase. In the case of HGF receptor (Met), phosphorylation of a single multifunctional site triggers a pleiotropic response involving multiple signal transducers. The synchronous activation of several signaling pathways is essential to coffering the distinct invasive growth ability of the HGF receptor (Ref.2).
Met receptor is a heterodimeric protein of 190 kDa, which consists of a 45-kDa extracellular Alpha-subunit linked by disulfide bonds to a 145-kDa Beta-subunit that spans the membrane and contains the catalytic tyrosine kinase domain. Binding of HGF induces Met dimerization and autophosphorylation of two Phosphotyrosine residues: PY1349 and PY1356 of the Beta subunit, generating a multidocking site at the C-terminus, which activates multiple signaling cascades (Ref.3). These phosphotyrosines mediate high-affinity interactions with various cytoplasmic effectors, which then transduce extracellular signals elicited by HGF to downstream targets. One of the major substrates of the activated HGF receptor tyrosine kinases is the adaptor protein GAB1 (GRB2-Associated Binding Protein-1). Phosphorylated GAB1 binds signal-relay molecules, such as the SH2-domain-containing proteins: SHP2 (Tyrosine Phosphatase-2), PI3K (Phosphatidylinositol-3 Kinase), PLC-Gamma (Phospholipase-C-Gamma), STAT3 (Signal Transducer and Activator of Transcription-3) and CrkL, through their SH2 domains. GAB1 interacts with CrkL, a protein with SH2 and SH3 protein interaction domains that couples to signaling further downstream (Ref.4). The actions of HGF on Pxn (Paxillin), DOCK1 (Dedicator of Cytokinesis-1) and Rap1 which alter cell motility are also mediated through GAB1. Via its SH3 domains, CrkL can associate with, and activate multiple effector proteins, like DOCK1 C3G (Guanine Nucleotide Releasing Protein C3G), a GDP GTP exchange factor for Rap1. C3G is implicated in the activation of Rap1, which further activates FAK (Focal Adhesion Kinase) and Pxn, associated with Itg (Integrin). The activation of FAK induces the formation of focal adhesions, a preliminary step to increased cell motility and tissue invasion by transformed cells, and Paxillin phosphorylation may also alter cell adhesion of Met transformed cells (Ref.5).
Activated Met can specifically recruit GRB2 (Growth Factor Receptor-Bound Protein-2), an adaptor protein that couples activated receptor tyrosine kinases to SOS (Son of Sevenless), promoting activation of Ras (Ref.6). The activation and inactivation of Ras are regulated by GEPs (Guanine Exchange Proteins) and GAPs (GTPase-Activating Proteins). The major human GEP for Ras is SOS, which is constitutively associated with GRB2. GAB1 contributes to Ras activation through activation of the SH2-domain-containing protein: SHP2. Activation of Ras by HGF results in activation of Raf1, followed by the subsequent threonine and tyrosine phosphorylation of cytoplasmic dual specificity kinases, MEK1 (MAPK/ERK Kinase-1) and MEK2 (MAPK/ERK Kinase-2). The MEKs in turn activate the extracellular signal-regulated kinases: ERK1 and ERK2. The activation of these MAPKs is required for HGF-elicited cell scattering and tubulogenesis (Ref.7). Major substrates for ERKs are the transcription factors Elk1 and Ets, which upon activation, up-regulate the expression of immediate early response genes, such as c-Fos. The ERKs also stimulate the stress-responsive transcription factors: c-Jun and c-Fos, found to be important in HGF-mediated survival. Regulation of Rac1 and CDC42 pathways in response to HGF all contribute to cytoskeletal rearrangement and the subsequent changes in cellular motility. HGF functions as a scattering factor for epithelial cells, and this ability is mediated through the activation of STAT3. Phosphorylation of STAT3 and the resulting nuclear signaling alters cellular transcription in addition to altering cell adhesion, proliferation and cell motility required for triggering differentiation for branching morphogenesis. Rac1 and CDC42, both are activated by phosphorylated Ras. DOCK1 also lies upstream of the Rac1 pathway (Ref.1 & 6). Activation of the Rac1 pathway and the CDC42 pathway contributes to the regulation of cytoskeleton, thus culminating in cell polarity and cell motility. Besides cell migration, activation of Rac1 HGF also contributes to cell survival and differentiation by activating the MEKK (MAP/ERK Kinase Kinases)-->MEK4/7-->JNK (c-Jun Kinase) pathway . JNK activates the c-Jun and c-Fos transcription factors. Activation of various transcription factors by HGF induces expression of several genes, involved in cell survival and cell cycle progression (Ref.8). For example, CDK6 (Cyclin-Dependent Kinase-6), Rb (Retinoblastoma) and p27/p27(KIP1) (Cyclin Dependent Kinase Inhibitor-p27) are expressed, which act as positive regulators of cell cycle progression. The anti-apoptotic gene COX2 (Cyclooxygenase-2) is also induced by HGF in a c-Jun- and c-Fos-dependent manner (Ref.9). COX2 expression by HGF inhibits the process of Anoikis (also known as Suspension-Induced Apoptosis), is a term used to describe apoptosis of epithelial cells induced by loss of matrix attachment. This process is important for maintaining normal cell and tissue homeostasis (Ref.10).
Protection of cells against DNA damage by HGF is mediated by a pathway from its receptor c-Met to PI3K through GAB1 to Akt and PAK1 (p21-Activated Kinase), resulting in enhanced DNA repair and decreased apoptosis (Ref.8). Activation of PAK1 also inhibits Anoikis. The PI3K pathway is responsible for cell scattering by inducing the loss of intercellular junctions and cell migration. Akt, the downstream target of PI3K, exerts its anti-apoptotic effects in a variety of ways, including phosphorylation and activation of IKKs (I-KappaB Kinases). This results in I-KappaB degradation and allows NF-KappaB to enter the nucleus and activate transcription of anti-apoptotic genes. Another mechanism whereby Akt functions to promote survival is through phosphorylation of BAD. Akt also phosphorylates Procaspase9 and inhibits its protease activity, thus suppressing activation of Procaspase3 and promoting cell survival as Caspase3 activity has a reverse correlation with Akt activity (Ref.7). Activation of the Met receptor also results in an increase in receptor-mediated activation of PLC-Gamma which catalyzes the generation of IP3 (Inositol 1,4,5-Trisphosphate) and DAG (Diacylglycerol) from PIP2 (Phosphatidylinositol 4,5-Bisphosphate), which act as second messenger molecules to mobilize intracellular Calcium and activate PKC (Protein Kinase-C) respectively. These signaling pathways act as important components of the cell survival and cell migratory response (Ref.11).
The Met tyrosine kinase is shown to be deregulated through gene amplification, overexpression, or activating point mutations in a number of human cancers, suggesting that the Met receptor plays an important role in human tumorigenesis (Ref.12). Anoikis is strongly suppressed by HGF through ERK and Akt-signaling pathway and because tumor cells lose matrix attachment during metastasis, conceptually anoikis resistance plays an important role in tumor progression and metastasis (Ref.13). Mutated forms of the HGF receptor are associated with oncogenesis and metastasis, making the HGF receptor a potential therapeutic target for cancer drugs. Changes in cell motility; cell shape, adhesion, resistance to apoptosis, and anchorage independent growth all contribute to the role of HGF in cancer (Ref.5 & 14). Several studies have shown that HGF and MET are Involved also involved in arterial repair and Atherogenesis (Ref.15). Recent studies on possible mechanisms of HGF gene therapy implicate the possible use of HGF as a powerful therapeutic agent against graft failure, restenosis, cardiomyopathy, cerebral vascular diseases, renal failure etc. (Ref.11).
References
- 1
- Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression.
- 2
- Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase.
- 3
- Tulasne D, Paumelle R, Weidner KM, Vandenbunder B, Fafeur V. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering.
- 4
- Fan S, Ma YX, Gao M, Yuan RQ, Meng Q, Goldberg ID, Rosen EM. The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair.
- 5
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more.
- 6
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses.
- 7
- Delehedde M, Sergeant N, Lyon M, Rudland PS, Fernig DG Hepatocyte growth factor/scatter factor stimulates migration of rat mammary fibroblasts through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways.
- 8
- Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways.
- 9
- Zeng Q, McCauley LK, Wang CY. Hepatocyte growth factor inhibits anoikis by induction of activator protein 1-dependent cyclooxygenase-2. Implication in head and neck squamous cell carcinoma progression.
- 10
- Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D, Wang CY. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFkappa B.
 关于我们
关于我们
