Hedgehog
发布时间:2019-12-10 12:03 来源:SABiosciences
- 通路
- 概述
Review
Controlled cell proliferation is a predominant theme in normal embryonic and post-embryonic development, and, in many instances, cell-type specification and cell proliferation are intimately coupled. Several secreted intercellular signaling proteins that behave as morphogens during pattern formation are also implicated in the regulation of the cell cycle. Hedgehogs (Hhs) are one such class of morphogens that regulate an enormous variety of developmental events in the fly and vertebrate embryo and plays a central role in several cancers.
The vertebrate Hh family is represented by at least three members: Dhh (Desert Hh), Ihh (Indian Hh) and Shh (Sonic Hh), two Patched homologs, Ptc1 (Patched-1) and Ptc2 (Patched-2); and three homologs of Ci (Cubitus interruptus, a 155 kDa cytoplasmic zinc finger protein), Gli1, Gli2 and Gli3 (Ref.1). Shh is the most extensively characterized vertebrate homolog, and is involved in morphogenesis of several organs including the eye, hair and lungs. It acts as both a short-range, contact-dependent factor and as a long-range, diffusible morphogen. Shh genes are highly conserved and have been identified within a variety of species, including human, mouse, frog, fish, and chicken. In the human embryo, Shh is expressed in the notochord, the floorplate of the neural tube, the gut, and in the developing limbs. Dhh and Ihh play more restricted roles: Dhh acts in the regulation of spermatogenesis and organization of the perineurium, which ensheaths peripheral nerves, and Ihh in coordinating proliferation and maturation of chondrocytes during development of the endochondral skeleton. Hh signals act as morphogens to induce distinct cell fates at specific concentration thresholds. In Drosophila, Hh patterns the segment, wing, leg, eye, and regions of the fly brain either directly, or through the recruitment of other signaling factors such as Dpp (Decapentaplegic) and Wg (Wingless) (Ref.2).
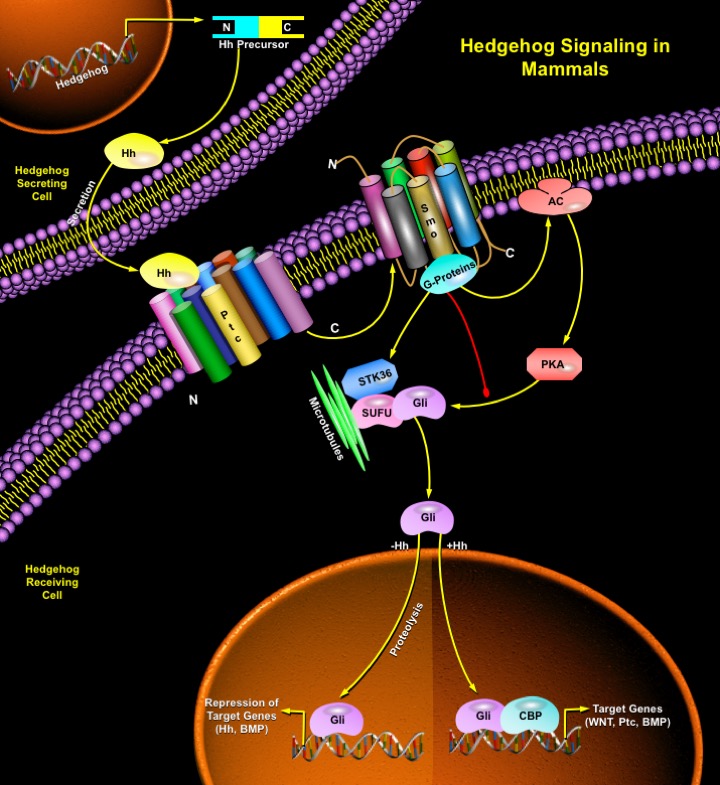
The Hh-signaling pathway comprises three main components: the Hh ligand; a transmembrane receptor circuit composed of the negative regulator Ptc plus an activator, Smo (Smoothened) a GPCR (G-Protein Coupled Receptor); and finally a cytoplasmic complex that regulates the Ci or Gli family of transcriptional effectors. Additional pathway components are thought to modulate the activity or subcellular distribution of these molecules. There is positive and negative feedback at the transcriptional level as the Gli1 and Ptc1 genes are direct transcriptional targets of activation of the pathway (Ref.3). Ptc, a twelve-pass membrane protein binds Hh ligand, and in the absence of ligand, Ptc interacts with and inhibits Smo, a seven-pass membrane protein. This repression culminates in a transcription factor, Ci (Ci75) in Drosophila and Gli in vertebrates acting as a transcriptional repressor. When Hh binds Ptc, its interactions with Smo are altered such that Smo is no longer inhibited. This leads to Ci/Gli protein entering the nucleus and acting as a transcriptional activator for the same genes it represses when Ptc is free to interact with and inhibit Smo. The determination of diverse cell fates by Shh signaling occurs by regulating the combination of Gli genes expressed in a cell. The transcriptional effects of Hh signaling are directed to particular target genes by the specificity of the Ci zinc fingers in DNA sequence recognition (Ref.4). The processing and nuclear import of Ci is regulated via a complex of Ci with the cytoplasmic members of the Hh signaling pathway, Cos2 (Costal-2; Cos-FlyBase), Fused (Fu) and SUFU (Suppressor of Fused). Cos2 tethers the Ci-containing complex to the microtubules. On Hh signaling, the complex is released from microtubules and full-length Ci enters the nucleus (Ref.5). Kinases including GSK3Beta (Glycogen Synthase Kinase-3Beta), Slimb and PKA (Protein Kinase-A) oppose activation of the Shh pathway by regulating the stability of intermediate signaling transcription factors of Hh pathway. SUFU interacts directly with Ci proteins, repressing Hh signaling. In the absence of Hh signal, Cos2 and SUFU binding to Ci prevent Ci activation and retain it in the cytoplasm. Most of Ci is available for cleavage in a process which is dependent upon its phosphorylation by the PKA and which involves Cos2 and Slimb. Uncleaved, full-length Ci is actively exported from the nucleus. Upon Hh reception, Fu is activated and acts on Cos2 and SUFU, alleviating their negative effect on Ci. As a result, Ci cleavage is reduced, Ci155 nuclear import overcomes its export and Ci is activated. Ci activation requires Cos2 and Fu to antagonize SUFU negative effect. Activated nuclear Ci interacts with the CBP (CREB Binding Protein) to fully activate the transcription of Hh target genes (Ref.6).
Since their isolation, members of the Hh family of intercellular signaling proteins have been recognized as key mediators of many fundamental processes in embryonic development. Their activities are central to the growth, patterning, and morphogenesis of many different regions within the body plans of vertebrates and insects, and most likely other invertebrates (Ref.7). Inactivation of Shh or components in its signal transduction pathway, such as Gli2 and Gli3, gives rise to various degrees of lung and foregut malformations, with fusion of lung lobes, hypoplasia and esophageal atresia or stenosis (Ref.1). Further, misregulation of Hh signaling in humans is associated with congenital malformations of the CNS (Central Nervous System, spina bifida, holoprosencephaly type 3, hpe3), head (cleft palate), and limb (syn- and polydactyly) and with a predisposition for developing a variety of tumors of the skin (basal cell carcinoma) and CNS (medulloblastoma, glioblastoma) (Ref.8). Hh signal transduction has been the focus of intense research over the past decade due to the central role it plays in development and its emerging biomedical relevance in areas ranging from regenerative medicine to oncology (Ref.3).
References
- 1
- Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations.
- 2
- McMahon AP. More surprises in the Hedgehog signaling pathway.
- 3
- Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and an
- 4
- Chen CH, von Kessler DP, Park W, Wang B, Ma Y, Beachy PA. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression.
- 5
- Vied C, Horabin JI. The sex determination master switch, Sex-lethal, responds to Hedgehog signaling in the Drosophila germline.
- 6
- Monnier V, Ho KS, Sanial M, Scott MP, Plessis A. Hedgehog signal transduction proteins: contacts of the Fused kinase and Ci transcription factor with the Kinesin-related protein Costal2.
- 7
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles.
- 8
- Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord.
 关于我们
关于我们