GSK3_Signaling
发布时间:2019-12-10 12:00 来源:SABiosciences
- 通路
- 概述
Review
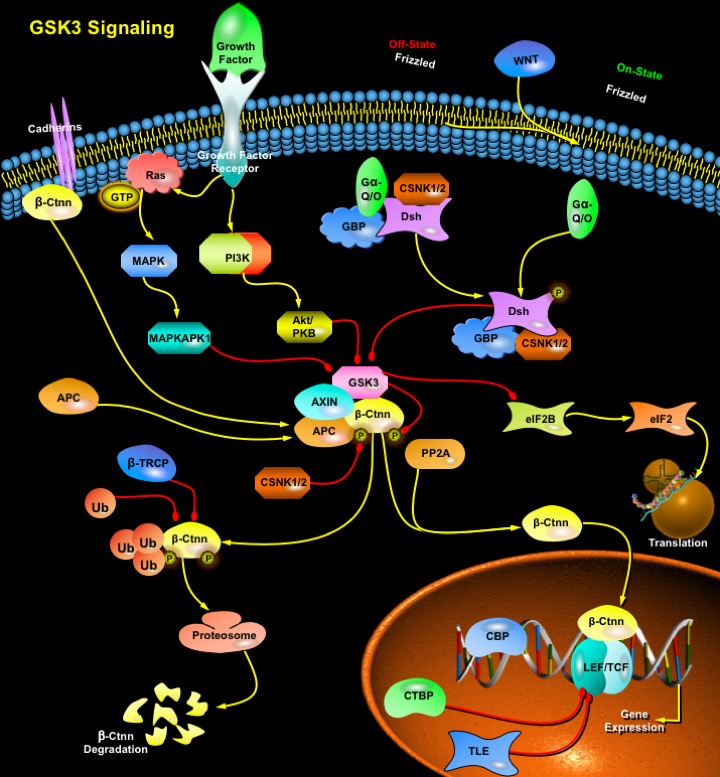
GSK3 (Glycogen Synthase Kinase-3) is a ubiquitously expressed, highly conserved serine/threonine protein kinase found in all eukaryotes. Identified originally as a regulator of glycogen metabolism, GSK3 acts as a downstream regulatory switch for numerous signaling pathways, including cellular responses to WNT, Growth Factors, Insulin, RTK (Receptor Tyrosine Kinases), Hedgehog pathways, and GPCR (G-Protein-Coupled Receptors) and is involved in a wide range of signal transduction cascades involving cellular processes, ranging from glycogen metabolism, cell development, gene transcription, protein translation to cytoskeletal organization, cell cycle regulation, proliferation and apoptosis. Unlike most protein kinases involved in signaling, GSK3 is active in unstimulated, resting cells and its activity is diminished during cellular responses. Another peculiarity compared with other protein kinases is its preference for primed substrates, that is, substrates previously phosphorylated by another kinase (Ref.1). Growth Factors and Insulin signaling inhibit the ability of GSK3 to act on pre-phosphorylated (primed) substrates by phosphorylating Ser9 of GSK3, which then blocks the interaction of GSK3 with the phosphate group on primed substrates (Ref.2). GSK3 is localized predominantly in the cytoplasm but is also found in the nucleus. Its subcellular localization is changed in response to stimuli.
There are two mammalian GSK3 isoforms encoded by distinct genes: GSK3-Alpha and GSK3-Beta. GSK3-Beta is particularly abundant in the CNS (Central Nervous System) and directly phosphorylates several neuronal MAPs (Microtubule-Associated Proteins), involved in microtubule stabilization (Ref.3). GSK3 is negatively regulated by of PI3K (Phosphatidylinositol 3-Kinase)-mediated activation of Akt/PKB (Protein Kinase-B) and by the WNT signaling pathway (Ref.5). In the absence of a WNT signal, GSK3 is a part of the multiprotein complex that includes the proteins AXIN (Axis Inhibitor), APC (Adenomatous Polyposis Coli), CSNK1 (Casein Kinase-1) and Beta-Ctnn (Beta-Catenin) which is localized at the membrane along with the Cadherins. These proteins help GSK3 to efficiently phosphorylate the signaling molecule Beta-Ctnn, thus targeting it for ubiquitination and subsequent proteosomal degradation. AXIN acts as a scaffolding protein in this complex, binding both GSK3 and Beta-Ctnn in a manner that brings them into close proximity, thus allowing GSK3 to phosphorylate Beta-Ctnn. AXIN therefore acts as a GSK3 activating protein. When GSK3 is active, it phosphorylates APC and Beta-Ctnn and stimulates interaction between Beta-Ctnn and Beta-TRCP (Beta-Transducin Repeat-Containing Protein), a regulator of E3 Ubiquitin Lligase, which degrades Beta-Ctnn in proteasomes (Ref.4 & 10). GSK3 phosphorylates a variety of substrates, other than Beta-Ctnn such as Glycogen Synthase and other metabolic enzymes, transcription factors CBP (CREB Binding Protein), c-Myc and c-Jun, and the translation initiation factors eIF2 and eIF2B (Ref.6). Phosphorylation of transcription factors by GSK3-Beta causes ubiquitination, nuclear exit, or decreases in the DNA binding, leading to decrease in nuclear transcription (Ref.4). In neuronal cells, GSK3 phosphorylate a number of MAPs, such as MAP2C, MAP1B and Tau. Phosphorylation of these proteins by GSK3 decreases their ability to stabilize microtubules (Ref.7).
Binding of the WNT molecules to Fz (Frizzled) receptors activates Dsh (Dishevelled) through G-proteins G-AlphaQ and G-AlphaO, which inhibits the activity of GSK3, thereby stabilizing Beta-Ctnn. CSNKI (Casein Kinase-I) also makes a complex with AXIN, GSK3 and Dsh and works as a positive regulator of the WNT signaling. Stabilization of Beta-Ctnn is associated with its translocation to the nucleus in presence of PP2A (Protein Phosphatase-2A) where it interacts with members of the LEF (Lymphoid Enhancer Factor)/ TCF (T-Cell Factor) and activates specific target genes (Ref.4). When Beta-Ctnn is absent, certain TCFs repress transcription by interacting with the corepressors TLE (Transducin-Like Enhancer) and CTBP (C-Terminal Binding Protein). Another GSK3 interacting molecule, GBP (GSK3 Binding Protein), and its mammalian homologue FRAT (Frequently Rearranged in Advanced T-Cell Lymphomas), binds to GSK3 and inhibits its phosphorylation of non-primed GSK3 substrates, including Beta-Ctnn. GBP/FRAT compete with AXIN for binding to GSK3, resulting in GSK3 inhibition (Ref.2). Numerous other stimuli also lead to inactivation of GSK3, including Growth Factors such as EGF (Epidermal Growth Factor) and PDGF (Platelet-Derived Growth Factor) that stimulate the GSK3-inactivating kinase p90RSK (also known as MAPKAPK1) through RasGTP-MAPK (Mitogen Activated Protein Kinase), activators of p70S6K (p70 Ribosomal S6 Kinase) such as amino acids, activators of cAMP-activated PKA (Protein Kinase-A) (Ref.1). GSK3 induce Caspase3 activation and activate the proapoptotic tumor suppressor gene, p53. It also promotes activation and translocation of the proapoptotic BCL2 (B-cell Lymphomal Leukaemia) family member, BAX (BCL2 Associated X-protein), which, upon aggregation and mitochondrial localization, induces CytoC (Cytochrome-C) release. Akt is one of the critical regulators of GSK3, and phosphorylation and inactivation of GSK3 may mediate some of the antiapoptotic effects of Akt (Ref.9).
GSK3 is a key regulator in several physiological processes, such as cell cycle, oncogenesis and apoptosis in neuronal cells and VSMC (Vascular Smooth Muscle Cells) during hypoxia (Ref.8). Increased cAMP levels promote survival of neuronal cells by inactivating GSK3 via a PKA–dependent mechanism (Ref.4). Many of the pathways that use GSK3 as a regulator have links to human diseases. GSK3 has been implicated in non-insulin-dependent Diabetes Mellitus and generation of NFT (Neurofibrilliary Tangles) associated with Alzheimer’s Disease (Ref.1) as well as several hallmarks of Alzheimer's Disease including neurodegeneration, reactive astrocytosis, microgliosis, and the formation of apoptotic bodies (Ref.8). The tangles are formed from hyperphosphorylated Tau. GSK3-Beta negatively regulates cardiac hypertrophy and cardiac development through its effect on WNT signaling (Ref.4). Recently, a number of potent and selective GSK3 inhibitors have been developed having several therapeutic uses, including the treatment of neurodegenerative disease, bipolar disorder, and inflammatory disease (Ref.8). However, the best-characterized inhibitor of GSK3 is lithium. Although inhibition of GSK3 may be desirable in one context (e.g. in preventing neuronal apoptosis), it could have serious implications for another—for example, it might accelerate hyperplasia by deregulating Beta-Ctnn. Given the involvement of GSK3 in many pathophysiological processes and diseases, GSK3 is a tempting therapeutic target (Ref.1).
References
- 1
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase.
- 2
- Ferkey DM, Kimelman D. Glycogen synthase kinase-3 beta mutagenesis identifies a common binding domain for GBP and Axin.
- 3
- Sayas CL, Avila J, Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms.
- 4
- Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development.
- 5
- Clodfelder-Miller B, De Sarno P, Zmijewska AA, Song L, Jope RS. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3.
- 6
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta.
- 7
- Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment.
- 8
- Woodgett JR. Judging a protein by more than its name: GSK-3.
- 9
- Loberg RD, Vesely E, Brosius FC 3rd. Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism.
- 10
- Jia J, Zhang L, Zhang Q, Tong C, Wang B, Hou F, Amanai K, Jiang J. Phosphorylation by double-time/CKIepsilon and CKIalpha targets cubitus interruptus for Slimb/beta-TRCP-mediated proteolytic processing.
 关于我们
关于我们