Growth_Hormone_Signaling
发布时间:2019-12-10 11:57 来源:SABiosciences
- 通路
- 概述
Review
Most aging individuals die from atherosclerosis, cancer, or dementia; but in the oldest old, loss of muscle strength resulting in frailty is the limiting factor for an individual's chances of living an independent life until death. Three hormonal systems show decreasing circulating hormone concentrations during normal aging: (i) estrogen (in menopause) and testosterone (in andropause), (ii) dehydroepiandrosterone and its sulphate (in adrenopause), and (iii) the growth hormone/IGF1 axis (in somatopause). Physical changes during aging have been considered physiologic, but there is evidence that some of these changes are related to this decline in hormonal activity. Science recognizes aging as a disease that can be reversed to a large degree by increasing GH (Growth Hormone) levels where they were in our young 20's. Biological aging is closely associated with a decline in the capacity for protein synthesis which has been hypothesized to contribute to the decline in tissue function and increased susceptibility to disease. GH and IGF1 (Insulin-Like Growth Factor-1) are two important anabolic hormones that regulate metabolic processes including protein synthesis in almost all tissues throughout the lifespan of mammals (Ref.1). GH is required for normal postnatal growth, having a critical role in bone growth as well as important regulatory effects on protein, carbohydrate, and lipid metabolism. The physiological effects of GH are brought about by the GHR (Growth Hormone Receptor) (Ref.2).
GH is a protein hormone composed of 191 amino acids that is secreted and synthesized by cells called somatrophs in the anterior pituitary gland, and has a profound effect on all the cells of the body, more than any other hormone. GH is produced in largest amounts during childhood and adolescence, the "peak" of our physical well being, and then gradually diminishes as we age. By age 61 our GH levels decrease to 80% less than when we were 21. The signs and symptoms of depleting GH levels in our bodies are the common signs of aging we all experience. They include: poor general health, increased body fat, increased anxiety and social isolation, lack of positive well-being. Low GH levels result in reduced energy and vitality, decreased muscle strength, increase in cholesterol, decreased bone mineral density etc. Defects in growth hormone signaling can result in dwarfism and decrease in growth hormone levels with age that play a role in the reduced function of the physiological systems (Ref.3).
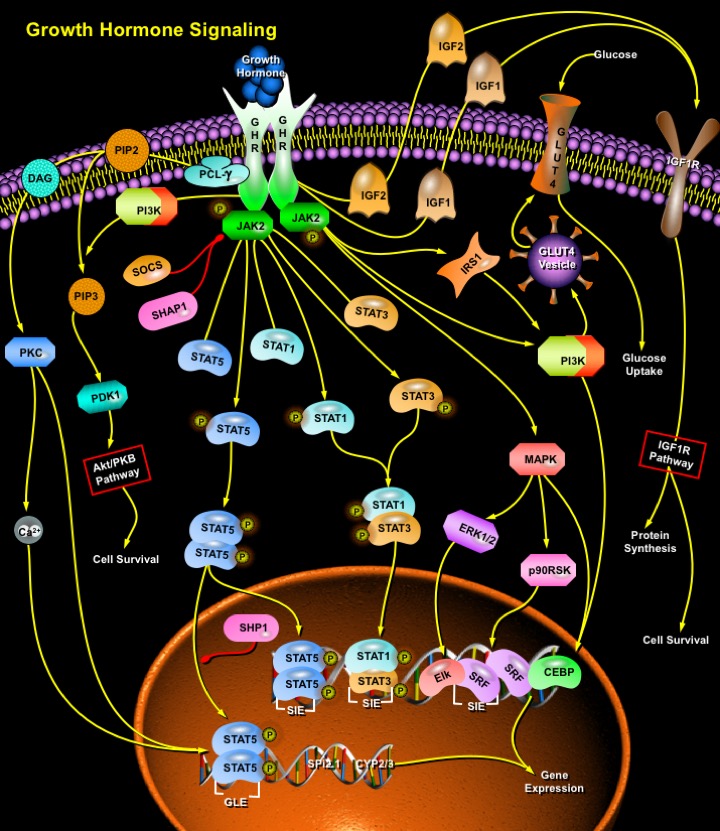
Multiple signaling pathways mediate the diverse effects of GH on growth and metabolism. Biologically active GH binds to two of its transmembrane receptors: GHRs, causes dimmerization of GHR, activation of the GHR-associated JAK2 (Janus-Family Tyrosine Kinase-2), and tyrosyl phosphorylation of both JAK2 and GHR (Ref.1). These events recruit and/or activate a variety of signaling molecules, including MAPKs (Mitogen-Activated Protein Kinases), IRS1 (Insulin Receptor Substrate-1), PI3K (Phosphatidylinositol- 3-Phosphate-Kinase), DAG (Diacylglycerol), PKC (Protein Kinase-C), Ca2+ (intracellular calcium), and STATs (Signal Transducers and Activators of Transcription). These signaling molecules contribute to the GH-induced changes in enzymatic activity, transport function, and gene expression that ultimately culminate in changes in growth and metabolism. Cross-talk among these signaling cascades in regulating specific genes suggests a GH-regulated signaling network. Activation of PI3K and IRS1 by GH signaling results in increased glucose uptake by effecting the translocation of GLUT4 (Glucose Transporter Protein-4) from an intracellular compartment to the plasma membrane. PI3K also activates the Akt/PKB Pathway through PDK-1 (Phosphoinositide Dependent Kinase-1) that culminates in cell survival (Ref.3).
STAT proteins 1, 3, and 5 are recruited to the GHR-Jak2 complex and become tyrosine phosphorylated. Further phosphorylation of STAT proteins at serine residues is followed by their dimerization and translocation to the nucleus (Ref.4). GH regulates two transcription factors associated with the c-fos SRE (Serum Response Element), SRF (Serum Response Factor), and Elk1 or another TCF (Ternary Complex Factor), which contribute to GH-dependent gene expression. GHRE (GH Response Element) in the Spi 2.1 promoter that contains two GAS sites is recognized by STAT5 (Ref.5). GLE (Gamma-Activated Sequence-Like Elements) in genes such as Spi 2.1, beta casein, and CYP 3A10/6-Beta-hydroxylase, bind the transcription factor STAT5 and can mediate reporter expression in response to GH. While STAT5 plays a prominent role in the regulation of genes containing GLE sequences by GH, binding of STAT1 and STAT3 to the SIE (Sis-Inducible Element) in response to GH contribute to the regulation of c-fos gene expression. STAT5 also participates in c-fos gene gene expression in a SIE dependent manner. GH-induced association of the GHR-JAK2 complex leads to activation of the Ras-MAPK pathway. Activated MAPKs ERK1 and ERK2 (Extracellular Signal Regulated Kinases) phosphorylate a TCF (e.g. Elk1), which leads to transcriptional activation via the SRE. GH may also regulate the phosphorylation of SRF via p90RSK (p90 Ribosomal S6 Kinase) (Ref.6). GH has also been reported to stimulate the synthesis and binding of the transcription factors CEBP-Beta (CCAAT/Enhancer-Binding Protein-Beta) and CEBP-Delta, which have been implicated in cell differentiation and proliferation. GH-dependent MAPK activation plays a role in the regulation of nuclear relocalization of C/EBP-Beta.
The actions of human GH are are also achieved through the stimulation of IGF (IGF1 and IGF2) production in target tissues. GHR dimmerization activates the synthesis and secretion of IGF1. The IGFs circulate, bound to specific IGFBPs (IGF Binding Proteins) and work in an autocrine, paracrine, or endocrine fashion by binding to specific receptors (Ref.2). In plasma, IGF1 binds to the soluble IGF1R (IGF1 Receptor). GH regulates the activity of IGF1 by increasing the production of binding proteins (specifically IGFBP-3 and another important protein called the acid-labile subunit) that increase the half-life of IGF1 from minutes to hours. Circulating proteases then act to break up the binding protein/hormone complex thereby releasing the IGF1 in a controlled fashion over time. GH may even cause target tissues to produce IGFBP3 increasing its effectiveness locally. At target cells, this complex activates signal-transduction pathways that result in the mitogenic and anabolic responses that lead to growth (Ref.5).
Factors like SOCS (Suppressor of Cytokine Signaling) and SHP1 (SH2-Containing Protein Tyrosine Phosphatase-1) play an important role in the down regulation of signaling by GH. During a GH response, SHP1 translocates to the nucleus and associates with phosphorylated STAT5, suggesting that it can participate in the dephosphorylation of nuclear STAT5. At the same time, SHP1 is also associated with JAK2 and appears to be involved in the attenuation of GH-activated JAK activity (Ref.5). GH is an anabolic hormone that induces positive nitrogen balance in intact animals and protein synthesis in muscle. At all ages, treatment of humans with human GH increases muscle size in GH-deficient individuals. Growth hormone enhances amino acid uptake into skeletal muscle, suggesting that this tissue is a primary target of the physiological effects of GH. The regulation of GH secretion and its action at target tissues is believed to be the most fundamental determinant of body size. While other growth factors have been discovered, GH seems to fit the role of the primary growth hormone. GH is regulated by nutrition and by the hormonal and genetic milieu that controls the timing and rate of growth. Exercise in humans is a well-known provocative stimulus for GH release. Growth hormone exerts lipolytic effects on fat and muscle, and circulating free fatty acids and glycerol levels rise following acute administration of GH. Many of the effects of GH on growth and metabolism are actually mediated indirectly via control of the synthesis of other growth factors (Ref.1).
References
- 1
- Butler AA, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles.
- 2
- Goodyer CG, Figueiredo RM, Krackovitch S, De Souza Li L, Manalo JA, Zogopoulos G. Characterization of the growth hormone receptor in human dermal fibroblasts and liver during development.
- 3
- Carter-Su C, Schwartz J, Smit LS. Molecular mechanism of growth hormone action.
- 4
- Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling.
- 5
- Bergad PL, Schwarzenberg SJ, Humbert JT, Morrison M, Amarasinghe S, Towle HC, Berry SA. Inhibition of growth hormone action in models of inflammation.
- 6
- Liao J, Hodge C, Meyer D, Ho PS, Rosenspire K, Schwartz J. Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element.
 关于我们
关于我们
