GPCR_Pathway
发布时间:2019-12-10 11:48 来源:SABiosciences
- 通路
- 概述
Review
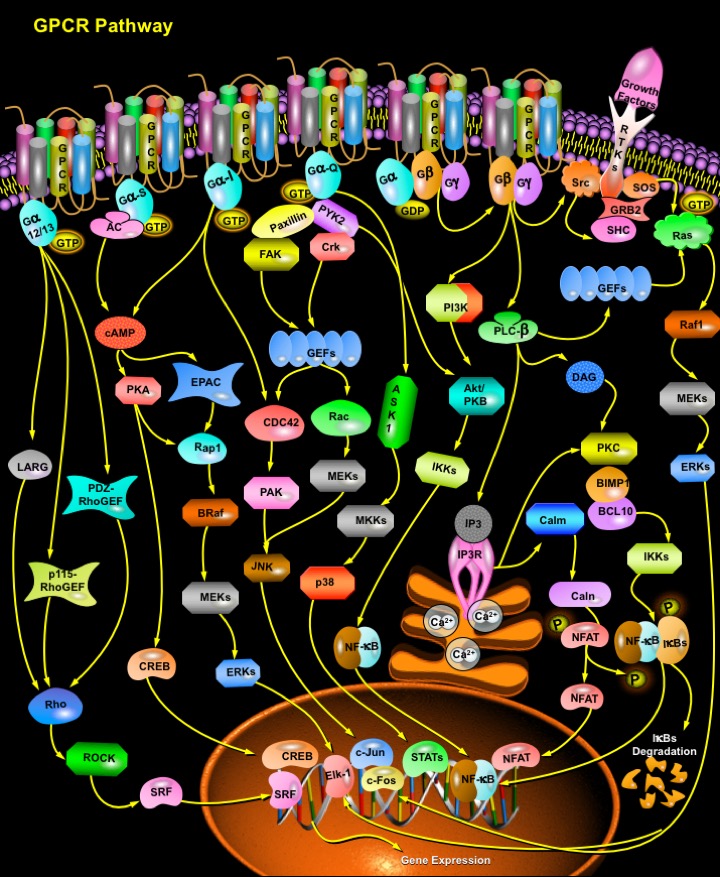
GPCRs (Guanine Nucleotide Binding–Protein Coupled Receptors) comprise large and diverse gene families in fungi, plants, and the animal kingdom. Also termed serpentine receptors, GPCRs are polytopic membrane proteins that share a common structure with seven transmembrane segments, but sequence similarity is minimal among the most distant GPCRs. Their principal function is to transmit information about the extracellular environment to the interior of the cell, and they do this by interacting with the G-proteins. GPCRs recognize a variety of ligands and stimuli including peptide and non-peptide hormones and neurotransmitters, chemokines, prostanoids and proteinases, biogenic amines, nucleosides, lipids, growth factors, odorant molecules and light. These receptors affect the generation of small molecules that act as intracellular mediators or second messengers, and can regulate a highly interconnected network of biochemical routes. The intracellular signaling pathways activated by GPCR signaling include cAMP (Cyclic Adenosine Monophosphate)/ PKA (Protein Kinase-A) pathway, Ca2+/PKC (Protein Kinase- C) pathway, Ca2+/NFAT (Nuclear Factor of Activated T-cells) pathway, PLC (Phospholipase- C) pathway, PTK (Protein Tyrosine Kinase) pathway, PKC/MEK (MAPK/ERK1) pathway, p43/p44MAPK (Mitogen Activated Protein Kinase) pathway, p38 MAP pathway, PI3K (Phosphoinositide-3 Kinase) pathway, NO-cGMP pathway, Rho pathway, NF-KappaB (Nuclear Factor-Kappa B) pathway and JAK (Janus Kinase )/ STAT (Signal Transducers and Activators of Transcription Factors) pathway (Ref.1).
Upon activation, GPCRs interact with their cognate G-proteins. G-proteins are heterotrimers (i.e., made of three different subunits) associated with the inner surface of the plasma membrane. The three subunits are: G-Alpha , G-Beta and G-Gamma. When signaling, they function in essence as dimers because the signal is communicated either by the G-Alpha subunit or the G-Beta-Gamma complex. Currently there are 20 known G-Alpha , 6 G-Beta , and 11 G-Gamma subunits. On the basis of sequence similarity, the G-Alpha subunits have been divided into four families: G-AlphaS, G-AlphaI, G-AlphaQ, and G-Alpha12/13 . These G-Alpha subunits regulate the activity of several second messenger-generating systems. In the inactive state, G-Alpha has GDP in its binding site. When a hormone or other ligand binds to the associated GPCR, an allosteric change takes place in the receptor. This triggers an allosteric change in G-Alpha causing GDP to leave and be replaced by GTP. GTP activates G-Alpha causing it to dissociate from G-Beta-Gamma (which remain linked as a dimer) (Ref.2).
The G-Alpha subunit has many different functions, depending on its isoform. G-alpha subunit consists of 4 isoforms: G-AlphaQ, G-AlphaS, G-AlphaI and G-Alpha12/13.G-AlphaQ family controls the activity of Phosphatidylinositol-specific Phospholipases, such as PLC-Beta, which hydrolyzes PIP2 (Phosphatidylinositol 4,5-Bisphosphate) to generate two-second messengers, IP3 (Inositol 1,4,5-Trisphosphate) and DAG (Diacylglycerol). IP3 and DAG in turn lead to an increase in the intracellular concentrations of free Ca2+ and the activation of a number of Protein Kinases, including PKC (Ref.3). G-AlphaQ, working through PKC and possibly directly, also appears to regulate various isoforms of PLD (Phospholipase-D). G-AlphaQ is reported to activate the transcription factor NF-KappaB through PYK2 (Proline-Rich Tyrosine Kinase-2). The members of the G-AlphaS family activate AC (Adenylyl Cyclase) leading to the production of cAMP in the cell. cAMP then binds to the regulatory subunit of PKA (Protein Kinase-A) leading to the dissociation of the catalytic subunits. Once the catalytic subunits of PKA dissociate, they become active. PKA then lead to the phosphorylation of the GPCR. Once the GPCR is phosphorylated, it can then couple to G-AlphaI instead of G-AlphaS. G-AlphaI family members can inhibit AC, thereby controlling the intracellular concentrations of cAMP. G-Alpha subunits of the G-AlphaI family, which includes G-AlphaI-1, G-AlphaI-2, G-AlphaI-3, G-AlphaI-O, transducin (G-AlphaI-T), and gustducin (G-AlphaI-gust), also activate a variety of Phospholipases and Phosphodiesterases, and promote the opening of several ion channels. G-AlphaI and G-AlphaI-O can regulate signals from c-Src to STAT3 and to the Rap pathways. Both G-AlphaI and G-AlphaQ-coupled receptors can potently stimulate MAPK activation (Ref.4).
G-Beta-Gamma Subunit of G-Proteins directly couples to at least four effector molecules: PLC- Beta, K+ channels, AC, and PI3K. Overexpression of G-Beta-Gamma subunit was found to be sufficient to stimulate MAPKs. Furthermore, stimulation of MAPK activity by co expressed G-Beta-Gamma dimers did not require PKC activation, but involved the activation of Ras. The small G-protein Ras becomes activated when its GEF is recruited to the membrane (via RTKs, FAK (Focal Adhesion Kinase), etc.). Once Ras binds GTP, it can then recruit the serine/threonine kinase Raf to the membrane. When Raf translocate to the membrane, it becomes activated and then phosphorylates the dual specificity kinase MEK. This leads to the activation of MEK, which then phosphorylates a critical tyrosine and threonine on ERK (Extracellular signal-Regulated Kinase). ERK then phosphorylates and activate other cellular proteins (like p90RSK) as well as translocate into the nucleus and phosphorylate/activate transcription factors (like Elk1) and leads to changes in gene expression and cell cycle progression. G-Beta-Gamma also binds to PLC-Beta, which facilitates its activation by G-AlphaQ, and it has been shown to enhance G-AlphaS activation of AC. Further, G-Beta-Gamma also interacts with ion channels and recruits BARK (Beta-Adrenergic Receptor Kinase) and PI3K-Gamma to the membrane. G-Beta-Gamma could lead to the activation of the non-receptor tyrosine kinase Src. Src then leads to the tyrosine phosphorylation of the adapter protein SHC. SHC then recruit the GRB2 (Growth Factor Receptor Bound Protein-2)-SOS (Son Of Sevenless) complex to the membrane via the SH2 domain of GRB2 binding to the Phosphotyrosine on SHC. SOS, a GEF for Ras, can then exchange the GDP bound to Ras to GTP. Once Ras binds GTP, it is then activated, and the ERK activation cascade is initiated. GPCRs that couple to G-AlphaS could also activate ERK via G-Beta-Gamma. The nature of the signaling pathways controlled by G-Alpha12 family of GTPases (Guanosine Triphosphatases) has just begun to be elucidated. G-Alpha12 has been reported to directly interact with a GTPase-activating protein for Ras, Ras-GAP, and BTK (Bruton's Tyrosine Kinase). These observations require confirmation and extension to establish the cellular consequences in native systems of these direct interactions. G-Alpha12 is thought to stimulate PLD, c-Src, and PKC by as-yet unidentified mechanisms. In many cases it appears that different members of the MAPK family, such as ERK5 or JNK (c-Jun NH2-terminal Kinase), are activated. This activation should lead to regulation of gene expression. G-Alpha13 directly interacts with and activates a guanine nucleotide exchange factor for the GTPase Rho, p115RhoGEF, and thus activates Rho, leading to a variety of effects that include regulation of the Na+-H+ exchanger. Through the activation of PYK2, G-Alpha13 may engage the PI3K pathway to activate the protein kinase Akt and regulate NF-KappaB. How G-Alpha13 activates PYK2 is currently not understood (Ref.5).
GPCRs mediate hormonal control of numerous signaling pathways. Many of these pathways are dynamically regulated. At the level of the receptor, regulation can occur via inhibition of GPCR/G-Protein coupling (desensitization), redistribution of cell surface receptors (trafficking), and receptor degradation (down-regulation). Two protein families, GRKs (G- protein-coupled Receptor Kinases) and Arrestins, play a critical role in regulating these processes. GRKs specifically phosphorylate the activated form of the receptor, which in turn promotes Arrestin binding. Arrestin binding sterically inhibits coupling of the GPCR to its respective G protein (a process termed receptor desensitization) and targets the receptor for internalisation via clathrin-coated pits. By regulating both the functional status and number of plasma membrane located GPCRs the GRKs play a pivotal role in modulating GPCR-mediated signal transduction. GPCRs are a pharmacologically important protein family with approximately 450 genes identified to date. Pathways involving these receptors are the targets of hundreds of drugs, including antihistamines, neuroleptics, antidepressants, and antihypertensives (Ref.6).
References
- 1
- Fang Y, Lahiri J, Picard L. G protein-coupled receptor microarrays for drug discovery.
- 2
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors.
- 3
- Gilchrist A, Li A, Hamm HE. G alpha COOH-terminal minigene vectors dissect heterotrimeric G protein signaling.
- 4
- Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors.
- 5
- Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction.
- 6
- Spiegel A. CELL SIGNALING: beta-Arrestin--Not Just for G Protein-Coupled Receptors.
 关于我们
关于我们
