Glucocorticoid_Receptor_Signaling
发布时间:2019-12-10 11:44 来源:SABiosciences
- 通路
- 概述
Review
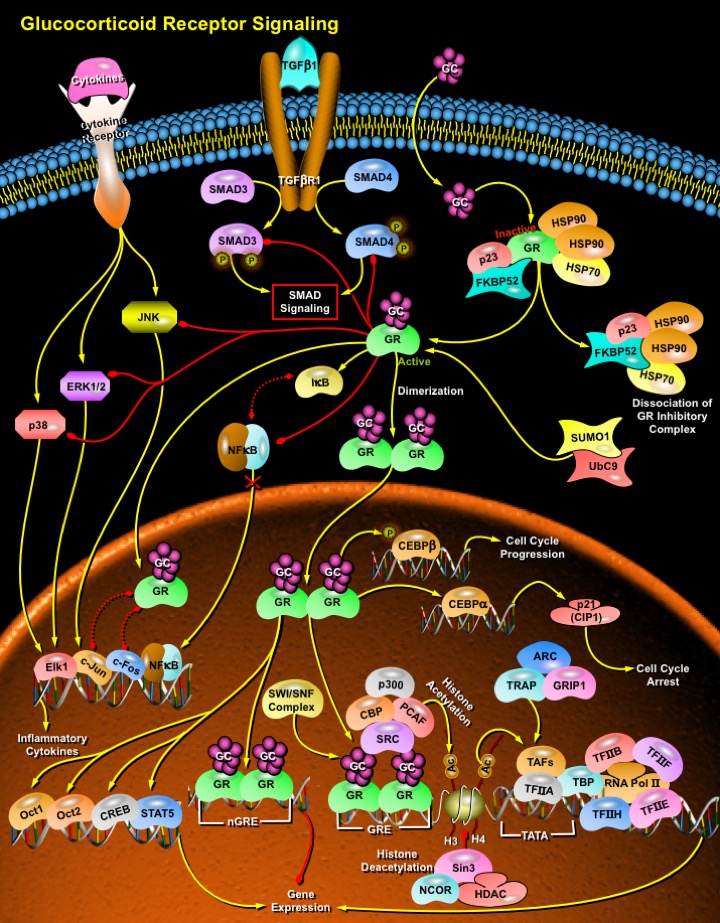
Our bones get more brittle with increasing age, and to add insult to injury, the most effective therapy for another problem that is associated with getting older, rheumatoid arthritis, often adds to the problem by causing bone resorption. The Glucocorticoid steroids, are the best available anti-inflammatories, and are used widely in the treatment of arthritis, as well as other inflammatory conditions such as dermatitis and autoimmune diseases. The Glucocorticoids, secreted by the Adrenal Cortex are powerful anti-inflammatory compounds due to their ability to inhibit all stages of the inflammatory response, from redness to wound healing to cell proliferation (Ref.1). They are powerful anti-inflammatory compounds that have the ability to inhibit all stages of the inflammatory response. They also have an essential role in cell metabolism and got the nomenclature, from their effect of raising the level of blood sugar (glucose) by stimulating gluconeogenesis in the liver: the conversion of fat and protein into intermediate metabolites that are ultimately converted into glucose. Cortisol (or Hydrocortisone) is the most important human Glucocorticoid. It is essential for life and regulates or supports a variety of important cardiovascular, metabolic, immunologic, and homeostatic functions. Corticosterone, another Glucocorticoid, helps in the regulation of the conversion of amino acids into carbohydrates and glycogen by the liver, and stimulates glycogen formation in the tissues. All the cellular responses to Glucocorticoids is attributed to their binding to the intracellular GR (Glucocorticoid Receptor) (Ref.2), that, in turn, translocates to the nucleus, that positively and negatively modulates gene expression through diverse mechanisms. The GR is the Glucocorticoid-activated member of the nuclear receptor superfamily of transcription factors. It mediates the immunosuppressive and anti-inflammatory activity of these ligands in multiple physiological systems, including the respiratory and central nervous systems. Belonging to the family of steroid hormones, Glucocorticoids are essential for development and survival of vertebrates (Ref.3).
Unbound GR is associated within the cytoplasm in an inactive oligomeric complex with some regulatory proteins such as the HSP90 (Heat Shock Protein-90 KD) which binds as a dimmer to the C-terminal domain, the HSP70 (Heat Shock Protein-70 KD), the p59 immunophilin, FKBP52 and the small p23 phosphoprotein. GRs are composed of several conserved structural elements, including a COOH-terminal ligand-binding domain (which also contains residues required for dimmerization and hormone-dependent gene transactivation), a nearby hinge region containing nuclear localization signals, a central zinc-finger-containing DNA-binding domain, and an NH2-terminal variable region important for ligand-independent gene transactivation. The interaction between HSP90 and GR is required to maintain the C-terminal domain in a favourable conformation for ligand binding (Ref.4). The Gucocorticoid hormone passes through the plasma membrane into the cytoplasm where it binds to the specific, high-affinity GR. The resulting complex is the non-DNA-binding oligomer of the GR in which the receptor is complexed with other proteins. Binding of hormone agonists releases GR from its interactions with the inhibitory complex, thus inducing a conformational change which results in unmasking of the receptor nuclear localization signal. Upon activation, GR thereby translocates to the nucleus and binds as a dimmer to DNA through its central domain, which is structurally characterized by a DNA binding motif (Ref.3). The stabilization and nuclear localization of GR is facilitated by its sumoylation by SUMO1 (Small Ubiquitin Related Modifier-1). The sumoylation process is catalyzed by the SUMO1-conjugating E2 enzyme Ubc9 (Ref.5). GR interacts either with DNA by targeting specific nucleotide palindromic sequences termed GRE (Glucocorticoid Response Elements) or nGRE (Negative GRE) (Ref.6). In particular, the dimmeric GR places its two DNA-binding fragments into adjacent major grooves of the DNA double helix in correspondence of appropriately spaced GRE half palindromes. Depending on the nature of the GRE, the overall process of GR binding can result in activation or repression of genes containing GR-binding sites (Ref.3).
Although the activity of the GR is often thought of simply in terms of direct gene transactivation, considerable cross-talk also occurs between the GR and a cohort of molecules to mediate their function as transcriptional regulators. GRs can interact with coactivator complexes including CBP (CREB-Binding Protein), p300, ACTR (Activator of Thyroid Hormone and Retinoid Receptors), SRC1 (Steroid Receptor Coactivator-1), and PCAF (p300/CBP Associated Factor) that possess HAT (Histone Acetyltransferase) activities, and the SWI/SNF complex which possesses ATPdependent chromatin remodeling activities (Ref.3 & 7). Acetylation of core histones alters nucleosomal packing to allow increased access of transacting factors and components of the basal transcriptional machinery to the local DNA. All these complexes may act in concert to relieve chromatin-mediated gene repression, with the TRAP (Thyroid Hormone Receptor Associated Protein)-GRIP (Glucocorticoid Receptor Interacting Proteins)-ARC (Activated Recruited Cofactor) complex functioning to recruit the core transcription machinery. The latter includes the TBP (TATA Box-Binding Protein), the TAFs (TBP Associated Factors), the general transcription factors TFIIA, TFIIB, TFIIE, TFIIF, TFIIH, and the enzyme, RNA Pol II (RNA Polymerase-II). The nuclear receptors can also interact with the corepressors NCoR (Nuclear Receptor Corepressor) and SMRT (Silencing Mediator of Retinoid and Thyroid Hormone Receptor) thus leading to the recruitment of the Sin3-HDAC (Histone Deacetylase) corepressor complex, possessing histone deacetylase functions. This corepressor complex can thereby inhibit gene transcription by counteracting the actions of the coactivator complexes containing histone acetyltransferase activities (Ref.2 & 8).
Alternatively, GR can also modulate the expression of genes through a GRE-independent mechanism, which is mediated in part through protein–protein interactions of GR with other sequence-specific DNA-binding factors or coactivators (Ref.9). The negative modulation of gene transcription operated by Glucocorticoids occurs through non genomic mechanisms (transrepression), mediated by inhibitory influences exerted by activated GR on the functions of several transcription factors. This contributes to the anti-inflammatory properties of the Glucocorticoids. Transrepression is due at least in part to direct, physical interactions between monomeric GR and transcription factors such as c-Jun-c-Fos and NF-KappaB (Nuclear Factor-KappaB), that synergistically coordinate the transcriptional activation of many genes involved in inflammatory diseases such as Asthma (Ref.10). In particular, the three main domains of GR may contribute to interact with the p65 subunit of NF-KappaB and with both Jun and Fos components of Activator Protein-1. The resulting reciprocal antagonism of the transcription factors engaged in these protein-protein associations causes an impairment of their transcriptional properties. However, Activator Protein-1, consisting of c-Jun homodimmers can also enhance GRE-mediated transactivation. On the other hand, Glucocorticoid-activated GR increases DNA-binding activity of CEBP-Beta via post-translational mechanisms involving phosphorylation at Thr(235) (Ref.11). GR can interact as a monomer, via direct protein-protein interactions, with transcription factors such as NF-KappaB and Activator Protein-1, activated by cytokines and other pro-inflammatory stimuli (Ref.4). The resulting mutual repression prevents both GR and the other transcription factors from binding to their respective DNA response elements. In addition, Glucocorticoids repress NF-KappaB-mediated activation of pro-inflammatory genes by reducing the levels of serine-2 phosphorylation of the carboxy-terminal domain of RNA Pol II, which is essential for the recruitment of this enzyme to the promoter region. Glucocorticoids also increase the transcription and synthesis of I-KappaB and thus may inhibit NF-KappaB by promoting its retention in the cytosol. Other products of Glucocorticoid inducible genes responsible for NF-KappaB inhibition include the two recently discovered proteins GILZ (Glucocorticoid-Induced Leucine Zipper) and GITR (Glucocorticoid-Induced Tumor Necrosis Factor Receptor Family-Related Gene), which play a crucial role in modulation of T-cell activation and apoptosis. GR can also cooperate with transcription factors, including octamer transcription factors Oct1 and Oct2; CREB (cAMP Response Element Binding Protein), and STAT5 (Signal Transducers and Activators of Transcription-5), to activate transcription. Competition for limiting co-activators of transcription is an important determinant of the fate of the cross-talk between the GR and other transcription factors. Both Activating Protein-1 and the GR are co-activated by CBP-p300, and in fact overexpression of CBP or p300 reverses the antagonism between Activator Protein-1 and the GR. Similarly, overexpression of CBP or SRC1 reverses the transcriptional antagonism between the GR and NF-KappaB (Ref.8 & 12).
Glucocorticoids downregulate cell proliferation by decreasing the expression of Cyclin-D1 and the phosphorylation of Rb (Retinoblastoma) protein and by activating p21(CIP1) (Cyclin Dependent Kinase Inhibitor-p21). The antiproliferative effect of Glucocorticoids is mediated by the GR and CEBP-Alpha, and both active transcription factors are required to induce the synthesis of p21(CIP1). In human cells, including lung fibroblasts, pulmonary and bronchial smooth-muscle cells, and peripheral-blood lymphocytes, the GR forms a complex with CEBP-Alpha, which then binds to the CCAAT DNA consensus sequence in the p21(CIP1) promoter (Ref.13). The Glucocorticoid signaling interacts with other signaling pathways activated by various cytokines, thus regulating diverse biological processes through modulating the expression of target genes. GR represses TGF-â transcriptional activation of the PAI-1 (Plasminogen Activator Inhibitor-1) and other genes in a ligand-dependent manner. Glucocorticoids inhibit the TGF-â-induced expression of ECM (Extracellular Matrix) proteins including Fibronectin and Collagen, and proteinase inhibitors such as tissue inhibitors of Metalloproteinase. GR inhibits transcriptional activation by both Smad3 and Smad4 C-terminal activation domains (Ref.14). The MAPKs (Mitogen-Activated Protein Kinases) play a key role in inflammatory cell types through transducing the response from proinflammatory cytokine receptors to the transcriptional apparatus. MAPK subgroups such as JNK regulate activation of the AP-1 complex required for proinflammatory gene expression. The MAPK p38 subgroup regulates the stability of mRNAs that encode the proinflammatory molecules TNF-Alpha, IL-6, IL-8, and VEGF (Vascular Endothelial Growth Factor). Negative regulation of the MAPK family by Glucocorticoids may be an additional mechanism by which the GR exerts its antiinflammatory effects (Ref.15). The MAPK subgroups JNK, ERK1, ERK2, and p38 are all targets of negative regulation by activated GRs. For example, Glucocorticoids destabilize the mRNA of the proinflammatory enzyme COX2 (Cyclooxygenase-2) by inhibiting the activity of p38 (Ref.16). The GR represses the MAPK family by inhibiting the phosphorylation step required for their activation. The defined molecular mechanism behind this inhibition has not been fully characterized and may be cell type and stimulus specific (Ref.9).
The therapeutic and prophylactic use of Glucocorticoids is widespread due to their powerful anti-inflammatory, antiproliferative and immunomodulatory activity (Ref.17). These are widely prescribed anti-inflammatory drugs, used to treat a wide variety of inflammatory diseases, including allergies, asthma, rheumatoid arthritis, and auto-immune diseases. Glucocorticoids enhance the production of other anti-inflammatory molecules such as IL-1RA (Interleukin-1 Receptor Antagonist), IL-10 (Interleukin-10), secretory leukocyte inhibitory protein and neutral endopetidase (Ref.2). Glucocorticoids are important mediators of the immune system and modulate the biological activities of inflammatory cytokines. The very effective control of airway inflammation exerted by Glucocorticoids in asthma is largely mediated by inhibition of the transcriptional activity of several different genes encoding pro-inflammatory proteins such as cytokines (IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-11, IL-13, TNF-Alpha, GMCSF, IFN-Gamma), chemokines (IL-8, RANTES, MIP-1a, MCP-1, MCP-3, MCP-4, Eotaxin), adhesion molecules (ICAM1, VCAM1, E-Selectin), and mediator-synthesizing enzymes (i-NOS, COX2, cytoplasmic PLA2) (Ref.9, 10 & 16). Glucocorticoids, acting through the GR, potently modulate immune function and are a mainstay of therapy for treatment of inflammatory conditions including allergies, asthma, rheumatoid arthritis; autoimmune diseases, leukemias and lymphomas (Ref.3). Common Glucocorticoids include prednisone, dexamethasone, and hydrocortisone. Hydrocortisone is used as an anti-inflammatory in the treatment of arthritis, as well as other inflammatory conditions such as dermatitis and autoimmune disease. While Glucocorticoids are widely used as drugs to treat various inflammatory conditions, prolonged Glucocorticoid use may have adverse side effects such as immunosuppression, fluid shifts, brain changes, and psychological changes. Physicians are therefore very cautious about prescribing these medications, especially for long periods of time. The search for novel Glucocorticoids with reduced side effects has been intensified by the discovery of new molecular details regarding the function of the Glucocorticoid receptor. These new insights may pave the way for novel, safer therapies that retain the efficacy of currently prescribed steroids (Ref.18).
References
- 1
- Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function.
- 2
- Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance.
- 3
- Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases.
- 4
- Davies TH, Ning YM, Sanchez ER. Differential control of Glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506.
- 5
- Tian S, Poukka H, Palvimo JJ, Janne OA. Small ubiquitin-related modifier-1 (SUMO-1) modification of the Glucocorticoid receptor.
- 6
- Ruegg J, Holsboer F, Turck C, Rein T. Cofilin 1 is revealed as an inhibitor of Glucocorticoid receptor by analysis of hormone-resistant cells.
- 7
- Grenier J, Trousson A, Chauchereau A, Amazit L, Lamirand A, Leclerc P, Guiochon-Mantel A, Schumacher M, Massaad C. Selective recruitment of p160 coactivators on Glucocorticoid-regulated promoters in Schwann cells.
- 8
- Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of Glucocorticoid signalling.
- 9
- Stellato C. Post-transcriptional and nongenomic effects of Glucocorticoids.
- 10
- Bladh LG, Liden J, Pazirandeh A, Rafter I, Dahlman-Wright K, Nilsson S, Okret S. Identification of target genes involved in the antiproliferative effect of Glucocorticoids reveals a role for nuclear factor-(kappa)B repression.
 关于我们
关于我们