FLT3_Signaling
发布时间:2019-12-10 11:41 来源:SABiosciences
- 通路
- 概述
Review
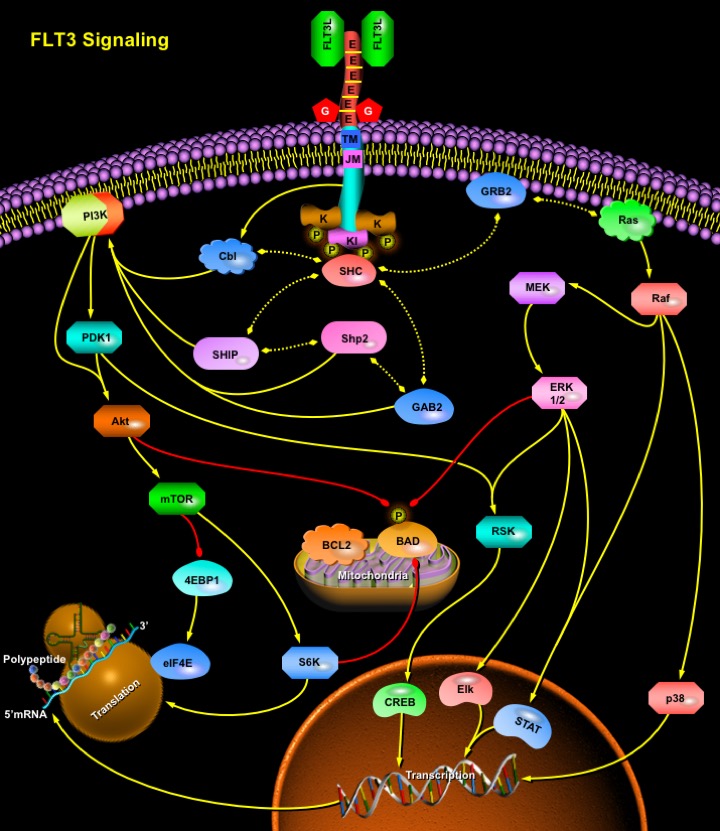
FLT3 (Fms-like Tyrosine Kinase-3), also known as FLK2 (Fetal Liver Kinase-2) and STK1 (human Stem Cell Kinase-1) was originally isolated as a hematopoietic progenitor cell-specific kinase, and belongs to the Class-III RTK (Receptor Tyrosine Kinase) family to which c-Fms, c-Kit, and the PDGFR (Platelet Derived Growth Factor Receptor) also belong (Ref.1). Normal expression of FLT3 is restricted to haemopoietic progenitor cells in the bone marrow, thymus and lymph nodes, but is also found on other tissues such as placenta, brain, cerebellum and gonads. Aberrantly expressed FLT3 is observed at high levels in a spectrum of hematologic malignancies including 70% to 100% of AML (Acute Myelogenous Leukemia), B-precursor cell ALL (Acute Lymphoblastic Leukemia), a fraction of T-Cell ALL, and CML (Chronic Myelogenous Leukemia) in lymphoid blast crisis (Ref.2). Human FLT3 is a 160 kDa, type I transmembrane glycoprotein originally cloned from a CD34+ stem cell library. The ligand for FLT3 (FLT3L) is expressed by marrow stromal cells and other cells and synergize with other growth factors to stimulate proliferation of stem cells, progenitor cells, dendritic cells, and natural killer cells. Interaction of FLT3 with its ligand results in receptor dimerization, autophosphorylation and the subsequent phosphorylation of cytoplasmic substrates that are involved in signaling pathways regulating the proliferation of pluripotent stem cells, early progenitor cells and immature lymphocytes (Ref.3). This interaction is influenced by other cytokines such as Kit ligand.
FLT3 contains five extracellular immunoglobulin-like domains (E), a TM (Transmembrane) domain, a JM (Juxtamembrane) domain and two tyrosine-kinase domains (K) that are linked through KI (Tyrosine-kinase Insert). Cytoplasmic FLT3 undergoes glycosylation (G), which promotes localization of the receptor to the membrane. Wild-type FLT3 remains as a monomeric, inactivated protein on the cell surface until FLT3L in a dimeric form, binds the receptor and induces receptor dimerization. FLT3 dimerization promotes phosphorylation of K, thereby activating the receptor and downstream effectors. The dimerized receptors are quickly internalized and degraded. Although the FLT3 signaling cascade has not been definitively characterized, the binding of FLT3L to FLT3 triggers the PI3K (Phosphatidylinositol 3-Kinase) and Ras pathways, leading to increased cell proliferation and the inhibition of apoptosis (Ref.4). PI3K activity is regulated through various interactions between FLT3, SHCs (SH2-containing sequence proteins) and other proteins, such as SHIP (SH2-domain-containing Inositol Phosphatase), SHP2 (SH2-domain-containing protein tyrosine Phosphatase-2), Cbl (a proto-oncogene) and GAB2 (GRB2 Binding protein). Activated PI3K stimulates downstream proteins such as PDK1 (3-Phosphoinositide-dependent Protein Kinase-1), Akt1/PKB (Protein Kinase-B) and the mTOR (mammalian Target of Rapamycin), which initiate the transcription and translation of crucial regulatory genes through the activation of S6K (p70 S6 kinase) and the inhibition of 4E-BP1 (eukaryotic initiation factor 4E-Binding Protein) (Ref.5). In addition, PI3K activation blocks apoptosis through phosphorylation of the pro-apoptotic BCL2 (B-Cell CLL/Lymphoma-2)-family protein BAD (BCL2 Associated Death Promoter). Activated FLT3 also associates with GRB2 (Growth Factor Receptor-Bound Protein-2) through SHC that activates Ras and stimulates downstream effectors such as Raf, MEK (MAPK/ERK Kinases), p38, ERK1/2 (Extracellular-signal Regulated Kinase), and the 90-kDa RSK (Ribosomal protein S6 Kinase). These downstream effectors activate CREB (cAMP Response Element Binding protein), Elk and STAT (Signal Transducer and Activators of Transcription), which lead to the transcription of FLT3 mRNA and translation to FLT3 protein involved in proliferation.
Functionally FLT3L shows marked activity on hematopoietic stem cells and thus maybe considered the stem cell growth factors. FLT3 is a promising molecular target for therapy of AML. The FLT3L/FLT3 system also plays an important role in lymphopoiesis. In particular, FLT3L synergize with IL-7 in inducing the proliferation of both B-Cell progenitors and later stage pro-B-Cells. FLT3L expressed constitutively by bone marrow fibroblasts, is a normal regulator of B-Cell lymphopoiesis, while cytokines produced by activated lymphocytes (such as IL-3) synergize with FLT3L in times of stress to accelerate B-Cell development. Two cytokines that down modulate FLT3 activity include TNF-Alpha (Tumor Necrosis Factor-Alpha) and TGF-Beta which block FLT3-induced hematopoietic activity, with TGF-Beta (Transforming Growth Factor-Beta) in particular demonstrating an ability to decrease FLT3 protein levels and reverse the FLT3L-induced decrease in the time that hematopoietic progenitors spend in the G1-phase of the cell cycle.
References
- 1
- Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P, Witte L, Burrow C, Ratajczak MZ, Gewirtz AM, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of
- 2
- Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.
- 3
- Lyman SD. Biology of flt3 ligand and receptor.
- 4
- Lavagna-Sevenier C, Marchetto S, Birnbaum D, Rosnet O. The CBL-related protein CBLB participates in FLT3 and interleukin-7 receptor signal transduction in pro-B cells.
- 5
- Zhang S, Broxmeyer HE. p85 subunit of PI3 kinase does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells.
 关于我们
关于我们
