Estrogen_Pathway
发布时间:2019-12-10 10:41 来源:SABiosciences
- 通路
- 概述
Review
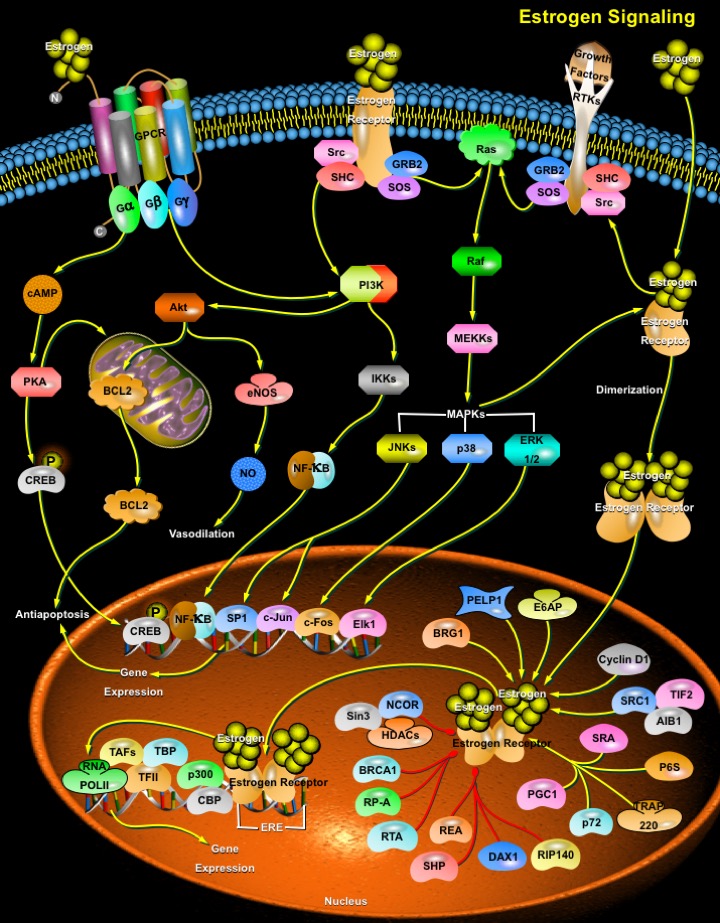
Estrogens play important roles in growth, development, reproduction, and maintenance of a diverse range of mammalian tissues. The physiological effects of estrogens are mediated by the intracellular ERs (Estrogen Receptors), which regulate transcription of target genes through binding to specific DNA target sequences. The ERs orchestrate both transcriptional and non-genomic functions in response to estrogens, xenoestrogens and signals emanating from growth factor signalling pathways. The pleiotropic and tissue-specific effects of estrogens are mediated by the differential expression of two distinct ER subtypes: ER-Alpha and ER-Beta, and their coregulators (Ref.1). The activities of a plethora of ER-interacting proteins converge to confer distinct functionalities on ERs, including the activation and repression of transcription, the integration of intracellular signaling pathways and the control of cell cycle progression. Both ERs are distributed widely in the body in both genders. ER-Alpha predominates in the uterus and mammary gland, whereas ER-Beta has significant roles in the central nervous, cardiovascular, and immune systems; urogenital tract, bone, kidney, and lungs (Ref.2). Typically, the majority of either ER-Alpha or ER-Beta is found in the cytoplasm and nucleus. However, small amounts (2%) can associate with the cell membrane.
The two mammalian ERs exhibit modular structures characteristic of the nuclear receptor superfamily. They are composed of three independent but interacting functional domains: the NH2-terminal transcriptional AF1 (Activation Function-1) domain, the DNA-binding domain, and the ligand-binding domain that contains a ligand-dependent transcriptional AF2 (Activation Function-2) domain (Ref.3). ERs integrate multiple signals both from ligands and intracellular signalling pathways to perform their functions in the nucleus and cytosol. The vasculature (like the reproductive tissue, bone, liver, and brain) has been recognized as an important target of estrogen action through rapid nongenomic effects and/or via the classic pathway (genomic effects) involving ERs (Ref.4). The classical pathways depend on direct interaction of estrogen with its receptor in the nucleus. Once activated, the ER complex can directly mediate gene transcription or interact with transcription factors to influence their activity. The nonclassical pathways work more rapidly and depend on the ability of estrogen to interact with either nonsteroid hormone receptors or steroid hormone receptors in the membrane. Both nonclassical pathways activate kinases that ultimately regulate transcription of specific genes (Ref.5).
The classical mechanism of steroid hormone action involves nuclear interactions of intracellular receptors, which are either cytoplasmic or nuclear. Binding of hormone to ER releases the receptor from an inhibitory complex with HSPs (Heat Shock Proteins) and triggers conformational changes that allow ER to bind the responsive elements in the target gene promoters (Ref.6). Subsequently, the receptor-ligand complex binds to the palindromic ERE (Estrogen Response Element) located in the target gene promoters, and stimulates gene transcription. Maximum transcriptional activity requires the concerted actions of the ligand-independent AF1 domain and the ligand-dependent AF2 domain. The transcriptional activity is also affected by a number of regulatory cofactors including chromatin-remodeling complexes, coactivators, and corepressors. Coactivators generally do not bind to the DNA but are recruited to the target gene promoters through protein-protein interactions with the ER. Examples of ER coactivators include, members of the p160/SRC (Steroid Receptor Coactivator) family: SRC1/NcoA1 (Nuclear Receptor Coactivator-1); NcoA2; NCOA3/AIB1/TRAM1/RAC3; the cointegrators: CBP (CREB-Binding Protein) and p300; and the family of CITED (CBP/P300-Interacting Transactivator, With Glu/Asp-Rich Carboxy-Terminal Domain) proteins. Corepressors like NCoR (Nuclear Receptor Co-Repressor) and MTA1 (Metastasis Associated-1) protein have been implicated in the transcriptional silencing. In addition, a few bifunctional coregulators such as PELP1 (Proline Glutamic Acid-Rich Nuclear Protein) also exist that can act both as coactivators and corepressors of ER (Ref.3). It is the relative balance of receptors, coactivator, and corepressor proteins, which is a critical determinant of the ability of this classical pathway to initiate responses. Since the relative concentrations of these molecules is cell specific, sex steroid hormones can have vastly different functions in different tissues of the same organism. A second mechanism of action for the classic pathway involves protein-protein interactions. In this pathway, ER-ligand complexes interact with transcription factors such as NF-KappaB (Nuclear Factor-KappaB), activator protein-1 and SP1 (Specific Protein-1) to influence gene transcription (Ref.1).
Estrogen receptors localized on the cell membrane and cytoplasm are also involved with the transduction of the nongenomic effects of estrogen, which are too rapid to be compatible with gene transcription and protein synthesis (Ref.7). Typically, these effects occur within seconds to minutes. These signaling cascades recruit second messengers including NO (Nitric Oxide), RTKs (Receptor Tyrosine Kinases), GPCRs (G-protein–Coupled Receptors), and protein kinases including PI3K (PhosphatidylInosiol-3-Kinase), serine-threonine kinase Akt, MAPK (Mitogen-Activated Protein Kinase) family members, and PKA and PKC (Protein kinases). Antiapoptotic role of estrogens is achieved through the activation of GPCRs and the Akt pathway. Activation of MAPK cascades leads to downstream cytoplasmic events or transcriptional events involving potentiation of AF1 activity (Ref.5). After binding ligand, ERs induce rapid phosphorylation of the adaptor proteins, Src and SHC (SH2 Containing Protein), resulting in a SHC–GRB2 (Growth Factor Receptor Binding Protein-2)–SOS complex formation. This leads to the subsequent activation of Ras, Raf, and MAPKs, including ERK-1/2 (Extracellular Signal Regulated Kinases), JNK (c-Jun N-terminal Kinase), and p38. They are then translocated to the nucleus and participate in gene transcription. Apart from this, MAPKs can directly catalyze the phosphorylation of serine 118 of the ER and increase its transcriptional efficiency. RSK (p90 Ribosomal-S6-Kinase), the downstream target of MAPK can also phosphorylate the ER, but at serine 167, an effect which increases its transcriptional efficiency. In breast and prostate cancer cells, Estrogen treatment activates the Src-Ras-ERK pathway, leading to cell cycle progression. Activated ERs elicit PI3K and Akt to activate eNOS (Nitric Oxide Synthase), which lead to enhanced NO release that may lead to vasodilation in the vasculature. In healthy blood vessels, the secretion of NO is vasculoprotective. Akt can also directly phosphorylate ER, resulting in enhanced ligand-independent transcription of estrogen-responsive genes (Ref.7).
Estrogens play a central role in reproduction, and, are regarded as the powerful female hormones that make a girl develop into a woman capable of reproduction. But now, estrogen is no longer viewed just as a female sex hormone but rather as a steroid hormone functioning in both females and males. In addition to their central role in reproduction, estrogens also affect the cardiovascular, skeletal, immune and nervous systems and play a role in the initiation and progression of breast cancer and osteoporosis (Ref.4). All these functions are effected, both through the action of the endogenous estrogens: E1 (Estrone), E2 (Estradiol/17-beta Estradiol) and E3 (Estriol); and, various syntehetic forms. Developmental exposure to high doses of exogenous E2 induces multiple persistent structural and functional abnormalities in the accessory sex glands. These include reduction in overall gland size; focal epithelial hyperplasia, metaplasia, and dysplasia; altered hormonal sensitivity; altered expression of ERs and AR (Androgen Receptor); alterations in stromal cell growth and function; disturbance of TGF-Beta (Transforming Growth Factor-Beta) signaling system; induction of protooncogenes; and inflammatory changes. In contrast, exposure to low doses of E2 has been reported to increase prostate size in adulthood (Ref.1). Multiple mechanisms participate in the regulation of estrogen-controlled genes, providing a wide spectrum of possibilities for development of drugs, including pure/mixed agonists or antagonists, known as: SERM (Selective Estrogen Receptor Modulators). Antiestrogens, such as Tamoxifen, are used as therapeutic agents for the treatment and possible prevention of breast cancer. Tamoxifen is believed to function as an antitumor agent by inhibiting the action of the ER in breast tissue (Ref.8).
References
- 1
- Moggs JG, Orphanides G. Estrogen receptors: orchestrators of pleiotropic cellular responses.
- 2
- Gustafsson JA. Novel aspects of estrogen action.
- 3
- Mishra SK, Mazumdar A, Vadlamudi RK, Li F, Wang RA, Yu W, Jordan VC, Santen RJ, Kumar R. MICoA, a novel metastasis-associated protein 1 (MTA1) interacting protein coactivator, regulates estrogen receptor-alpha transactivation functions.
- 4
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling.
- 5
- Lorenzo J. A new hypothesis for how sex steroid hormones regulate bone mass.
- 6
- Knoblauch R, Garabedian MJ. Role for Hsp90-associated cochaperone p23 in estrogen receptor signal transduction.
- 7
- Simoncini T, Rabkin E, Liao JK. Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3-kinase in endothelial cells.
- 8
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites.
 关于我们
关于我们
