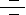ERK_Signaling
发布时间:2019-12-10 10:37 来源:SABiosciences
- 通路
- 概述

Review
The MAPK (Mitogen-Activated Protein Kinase) pathway is one of the primordial signaling systems that nature has used in several permutations to accomplish an amazing variety of tasks. It exists in all eukaryotes, and controls such fundamental cellular processes as Proliferation, Differentiation, Survival and Apoptosis. Mammalian MAPK can be divided into four groups based on their structure and function: ERKs (Extracellular signal-Regulated Kinases), p38MAPKs, JNKs (c-Jun NH2-terminal Kinases) and ERK5 (Extracellular signal-Regulated Kinase-5) or BMK. Activation of these MAPKs occurs through a cascade of upstream kinases; a MAPKKK (MAPK Kinase Kinase) first phosphorylates a dual-specificity protein kinase MAPKK (MAPK Kinase), which in turn phosphorylates the MAPK. This set-up provides not only for signal amplification, but, maybe even more importantly, for additional regulatory interfaces that allow the kinetics, duration and amplitude of the activity to be precisely tuned. ERK, a member of the MAPK family, have been established as major participants in the regulation of cell growth and differentiation, but when improperly activated contribute to malignant transformation. ERK1 and 2 form a central component in the MAPK cascade. The MAPK/ERK signaling cascade is activated by a wide variety of receptors involved in growth and differentiation including GPCRs (G-Protein Coupled Receptors), RTKs (Receptor Tyrosine Kinases), Integrins, and Ion channels. The specific components of the cascade vary greatly among different stimuli, but the architecture of the pathway usually includes a set of adaptors like SHC, GRB2 (Growth Factor Receptor Bound protein-2), Crk, etc. linking the receptor to a GEF (Guanine nucleotide Exchange Factor) like SOS (Son of Sevenless), C3G, etc. transducing the signal to small GTP binding proteins (Ras, Rap1), which in turn activate the core unit of the cascade composed of a MAPKKK (Raf), a MAPKK (MEK1/2 (MAPK/ERK Kinase-1/2)) and MAPK (ERK). An activated ERK dimer can regulate targets in the cytosol and also translocate to the nucleus where it phosphorylates a variety of transcription factors regulating gene expression (Ref.1 & 2).
GPCRs constitute a superfamily of Plasma membrane receptors. Members of this family include receptors for many Hormones, Neurotransmitters, Chemokines and Calcium ion, as well as sensory receptors for various odors, and bitter and sweet tastes. GPCR play an important role in activation of ERKs. Stimulation of the GN-AlphaI (Guanine Nucleotide Binding Protein-Alpha Inhibiting Activity Polypeptide)-coupled Neuropeptide Y1 and GN-AlphaQ (Guanine Nucleotide-Binding Protein-Alpha-Q) -coupled Muscarinic M1 Acetylcholine Receptors lead to the activation of ERK. When the GPCR becomes activated, it leads to the exchange of GDP for GTP on the GN-Alpha subunit. Upon activation, GN-AlphaI or GN-AlphaQ subunits are separated from GN-Beta (Guanine Nucleotide-Binding Protein-Beta) and GN-Gamma (Guanine Nucleotide-Binding Protein-Gamma) subunits and are converted to their GTP bound states that exhibit distinctive regulatory features on the nine tmACs (Transmembrane Adenylate Cyclases) in order to regulate intracellular cAMP (Cyclic Adenosine 3',5'-monophosphate) levels. cAMP activate Rap1A (Ras-Related Protein-1A) and Rap1B (Ras-Related Protein Rap1B) through EPAC (Exchange Protein Activated by cAMP)-dependent pathway. cAMP activates cAMP-GEFI (cAMP-Regulated Guanine Nucleotide Exchange Factor-I)/EPAC1 and cAMP-GEFII (cAMP-Regulated Guanine Nucleotide Exchange Factor-II)/EPAC2 that in turn activate Rap1A and Rap1B, respectively. Rap1A and Rap1B then forms an active complex with BRaf (v-Raf Murine Sarcoma Viral Oncogene Homolog-B1) for MEK1/2 activation finally resulting in ERK1/2 activation. cAMP may also activate PKA (Protein Kinase-A), which may further activate Rap and thus BRaf. On the other hand, PKA also inactivates C-Raf. GN-Alpha also directly activate PI3K (Phosphatidylinositide-3 Kinase) and c-Src and modulate their activity through stimulation of Ras under the influence of many extracellular factors. Rac is also a key downstream target/effector of PI3K. A new mechanism have been identified that regulates MEK1-ERK interactions and is dependent on Rac and PAK (p21-Activated Kinase). Besides GN-Alpha subunit, GN-Beta Gamma complex can also lead to activation of ERK. GN-Beta and GN-Gamma subunits when activated separate from GN-alpha subunit and it further activates PKC (Protein Kinase-C) via PLC-Beta (Phospholipase-C-Beta). PLC-Beta converts PIP2 (Phosphatidylinositol 4,5-bisphosphate) to DAG (Diacylglycerol), which activates PKC. PKC once formed can activate ERKs via Ras, Raf and MEKs. PKC also activates Src-PYK2 (Proline-Rich Tyrosine Kinase-2) complex which activates GRB2, which is also involved in ERK activation (Ref.3 & 4).
The canonical ERK MAPK cascade is also stimulated upon the binding of extracellular Growth factors to their respective transmembrane RTKs (Receptor Tyrosine Kinases). The subsequent auto-phosphorylation of the cytoplasmic tails of the receptor on tyrosine leads to the tyrosine phosphorylation of the adapter protein SHC. SHC can then recruit the GRB2-SOS complex to the membrane via the SH2 domain of GRB2 binding to the phosphotyrosine on SHC. SOS, a GEF for Ras, can then exchange the GDP bound to Ras to GTP. Once Ras binds GTP, it can then recruit the serine/threonine kinase Raf to the membrane. When Raf translocate to the membrane, it becomes activated and then phosphorylates the dual specificity kinase MEK. MEK binds and restricts inactive ERK to the cytosol. The MEK and ERK complex dissociates when MEK is activated and phosphorylates ERK. The ERK may then dimerize and this dimerization is apparently required for ERK to translocate into the nucleus by an active functions. Growth Factors may also activate ERK through PLC-Gamma and PKC. Integrins also play an important role in regulating the efficiency of the RTK/Ras/ERK pathway. Integrin regulation occurs at three (or more) different loci within the ERK pathway. First, in some cell systems, Integrin engagement with ligands enhances the activation and autophosphorylation of the RTKs. Secondly, Integrin engagement enhances the efficiency of the cytoplasmic cascade comprising Raf1, MEK and ERK. Finally, Integrin engagement is necessary for trafficking of activated ERK from the cytoplasm to the nucleus. FAK (Focal Adhesion Kinase) is a major nonreceptor tyrosine kinase activated after Integrin-mediated adhesion to ECM (Extracellular Matrix) proteins such as FN (Fibronectin). Interaction between FAK and the cytoplasmic tail of Beta1 Integrins results in autophosphorylation of FAK tyrosine 397 (FAK pY397) that can lead to stimulation of a cell-signaling cascade that ultimately activates the Ras/MAPK/ERK pathway. In addition to FAK, members of the Src family of nonreceptor protein-tyrosine kinases also associate with focal adhesions and are involved in Integrin signaling. Interestingly, Src and FAK appear to function in association with each other as a result of the binding of the Src SH2 domain to an autophosphorylation site of FAK. Src then phosphorylates additional sites on FAK. Tyrosine phosphorylation of FAK creates binding sites for the SH2 domains of other downstream signaling molecules, including PI3K and Rac. A key target of Rac is the protein-serine/threonine kinase PAK. Rac and CDC42 (Cell Division Cycle-42) can synergize with Raf to promote activation of the ERKs through mechanisms involving PAK1 phosphorylation of the MEK1 proline-rich sequence and PAK3 phosphorylation of Raf1. PAK3 can phosphorylate Raf1, enhancing Raf1 activation. Raf1 finally activates ERK1/2 via MEK1/2. The non-receptor tyrosine kinase PYK2 appears to function at a point of convergence of Integrins and certain GPCR signaling cascades. PYK2 acts with Src to link GN-AlphaI- and GN-AlphaQ-coupled receptors with GRB2 and SOS to activate the ERK signalling pathway (Ref. 5 & 6).
TCR (T-Cell Receptor)-CD3 complex also plays an important role in regulating ERK pathways in T-Cells. VHR is particularly interesting from a TCR signalling point of view because it accumulates at the T-Cell/APC (Antigen-Presenting-Cell) contact site, where it is phosphorylated at Tyr-138 by ZAP70 (Zeta-Chain-Associated Protein Kinase). This phosphorylation is required for VHR to inhibit the ERK MAPKs, giving ZAP70 an unanticipated control over MAPK dephosphorylation, in addition to its role as upstream activator of the Ras/Raf/MEK pathway. Other negative regulators of ERK pathway include PP2A (Protein Phosphatase-2A) and MKPs (MAPK Phosphatase). PP2A inhibits ERK pathway by dephosphorylating MEKs and is involved in the control of many cellular functions including metabolism, transcription, translation, RNA splicing, DNA replication, cell cycle progression, transformation, and apoptosis. The mammalian MAPK signaling system employ scaffold proteins, in part, to organize the MAPK signaling components into functional MAPK modules, thereby enabling the efficient activation of specific MAPK pathways. The ERK scaffold protein KSR (kinase suppressor of Ras) binds ERK, its direct activator MEK and Raf. In unstimulated cells KSR is mainly cytoplasmic but translocates to the Plasma membrane after growth factor stimulation, thus targeting MEK and ERK to the plasma membrane. A second targeting protein, p14, targets ERK2 to an endosomal location through its interaction with MP1 (MAPKK1-Interacting Protein-1), an adaptor protein that binds MEK and ERK. In addition, MEKK1 (MAP/ERK Kinase Kinase-1) can serve both as a scaffold and as MAPKKK, interacting specifically with MAPKK and MAPK (Ref.5, 7 & 8).
ERK once activated can either translocate to the nucleus to phosphorylate and activate transcription factors, while other pools of activated ERK phosphorylate a number of cytoplasmic targets. Cytosolic substrates for ERK include several pathway components involved in ERK negative feedback regulation. Multiple residues on SOS are phosphorylated by ERK following growth factor stimulation. SOS phosphorylation destabilizes the SOS-GRB2 complex, eliminating SOS recruitment to the plasma membrane and interfering with Ras activation of the ERK pathway. Negative feedback by ERK also occurs through direct phosphorylation of the EGF (Epidermal Growth Factor) receptor at Thr669. Finally, ERKs have also been demonstrated to negatively regulate themselves by phosphorylating MKPs (MAP Kinase Phosphatases) , which reduces the degradation of these phosphatases through the Ubiquitin-directed Proteasome complex. ERK also activate MNKs (MAPK-Interacting Kinases) by phosphorylation at Thr197 and Thr202. MNKs upregulate eIF4E (eukaryotic initiation factor-4E) through phosphorylation at Ser209 and play an important role in translation or they may also phosphorylate PLA2 (Phospholipase-A2). ERK1 and ERK2 regulate transcription indirectly by phosphorylating the RSKs (90 kDa Ribosomal protein S6 Kinases). Active RSKs appear to play a major role in transcriptional regulation, translocating to the nucleus and phosphorylating such factors as the product of proto-oncogene c-Fos at Ser362, SRF (Serum Response Factor) at Ser103, and CREB (Cyclic AMP Response Element-Binding protein) at Ser133. p90RSK also play an important role in cell survival by phosphorylating BAD (Bcl2-Antagonist of Cell Death). Another important cytoplasmic target of ERK is IKK-Alpha (I-KappaB Kinase-Alpha). IKK-Alpha phosphorylates I-KappaB-Alpha, which leads to ubiquitination and then leads to the degradation of I-KappaB-Alpha by the Proteosome, resulting in the translocation of NF-KappaB to the nucleus. In the nucleus it binds to its consensus sequence (5'-GGGACTTTC-3') and positively regulates the transcription of genes involved in immune and inflammatory responses, cell growth control, and apoptosis (Ref.9, 10 & 11).
Upon phosphorylation, nuclear translocation of ERK1 and ERK2 is critical for both gene expression and DNA replication induced by growth factors. In the nucleus, ERK phosphorylates an array of targets, including transcription factors and a family of RSK-related kinases, the MSKs (Mitogen- and Stress-activated protein Kinases). MSKs phosphorylate and activate the AP1 component ATF1 (Activating Transcription Factor-1) at Ser63, and may be more important in vivo than RSKs in CREB phosphorylation at the activating Ser133. MSKs were also found to phosphorylate Histone H3 at Ser10 and Ser28, and the HMG14 (High-Mobility-Group protein-14) at Ser6, facilitating the rapid induction of immediate early genes following mitogenic stimulation. Probably the best-characterized transcription factor substrates of ERKs are TCFs (Ternary Complex Factors), including Elk1, which is directly phosphorylated by ERK1 and ERK2 at multiple sites, including the activating Ser383. Upon complex formation with SRF, phosphorylated TCFs transcriptionally activate the numerous Mitogen-inducible genes regulated by SREs (Serum Response Elements). TCFs Sap1 and Sap2 are also phosphorylated by ERK, as are other Ets family members. Another direct target of ERK is the product of proto-oncogene c-Myc, a short-lived transcription factor involved in multiple aspects of growth control. Following phosphorylation at Thr58 and Ser62 within its transactivation domain, Myc activates transcription as a heterodimeric partner with Max. ERK can also phosphorylate CREB directly as well as AP1 components c-Jun and c-Fos. ERK1/2 also phosphorylates MLCK (Myosin Light Polypeptide Kinase), Capn (Calpain), Pax6 (Paxillin-6) and FAK that play important role in Cytoskeletal rearrangement. Other ERK targets include STAT1/3 (Signal Transducer and Activator of Transcription-1/3) and ESR (Estrogen Receptor). Finally, it can be concluded that ERKs are involved in the regulation of important neuronal functions, including neuronal plasticity in normal and pathological conditions. The kinetics and localization of ERK are intrinsically linked, in that the duration of ERK activation dictates its subcellular compartmentalization and/or trafficking. The latter, in turn, dictates whether ERK-expressing cells would enter a program of cell death, survival or differentiation. With aberrations in the ERK cascade implicated in a high proportion of human cancers, many emerging therapies target proteins in the pathway. As these candidate therapies against ERK signaling components undergo development and enter trials, reagents that monitor their targets' inhibition are critical for future success (Ref.12, 13 & 14).
References
- 1
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation.
- 2
- Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death.
- 3
- Osmond RI, Sheehan A, Borowicz R, Barnett E, Harvey G, Turner C, Brown A, Crouch MF, Dyer AR. GPCR screening via ERK 1/2: a novel platform for screening G protein-coupled receptors.
- 4
- Ginnan R, Guikema BJ, Singer HA, Jourd
- 5
- Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival.
- 6
- Sundberg-Smith LJ, Doherty JT, Mack CP, Taylor JM. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation.
- 7
- Puente LG, He JS, Ostergaard HL. A novel PKC regulates ERK activation and degranulation of cytotoxic T lymphocytes: Plasticity in PKC regulation of ERK.
- 8
- Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis.
- 9
- Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase.
- 10
- Parra JL, Buxade M, Proud CG. Features of the catalytic domains and C termini of the MAPK signal-integrating kinases Mnk1 and Mnk2 determine their differing activities and regulatory properties.
 关于我们
关于我们