Epithelial_Tight_Junctions
发布时间:2019-12-10 10:25 来源:SABiosciences
- 通路
- 概述
Review
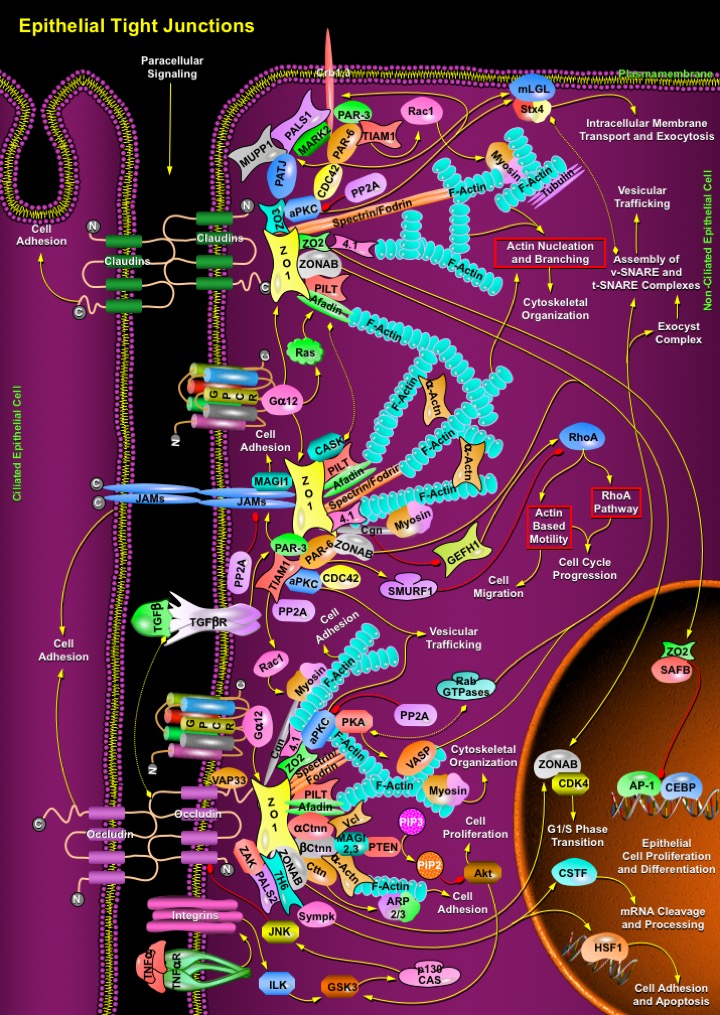
Epithelia in multicellular organisms constitute the frontier that separates the individual from the environment. Epithelia are sites of exchange as well as barriers, for the transit of ions and molecules from and into the organism. Epithelial cells achieve this by providing cellular borders that cover external and internal surfaces throughout the body. Complexes between adjacent cells include Gap Junctions, Desmosomes, Adherens Junctions (AJs) and Tight Junctions (TJs). Such junctions are quite essential for the modulation of paracellular permeability in various epithelia. Vertebrate epithelial cells exhibit Tight Junctions that lie apical to Adherens Junctions. Tight Junctions have an organizing role in epithelial polarization and establish an apico-lateral barrier to the diffusion of solutes through the intracellular space (gate function). They also restrict the movement of lipids and membrane proteins between the apical and the basolateral membrane (fence function). Tight Junctions are highly ordered membrane contact sites or ‘kissing points’, comprising a network of intra-membrane fibrils (Ref.1). They comprise at least four types of transmembrane proteins, including Occludins, Claudins, JAMs (Junctional Adhesion Molecules) and Crb (Crumb), and a number of cytoplasmic peripheral proteins. Whereas the transmembrane proteins mediate cell-cell adhesion, the cytosolic Tight Junction plaque contains various types of proteins (e.g. PDZ proteins, such as the ZO (Zona Occludens) family) that link Tight Junction transmembrane proteins to the underlying cytoskeleton. These adapters also recruit regulatory proteins, such as protein kinases, phosphatases, small GTPases and transcription factors, to the Tight Junctions. As a result, structural (Actin and Spectrin) and regulatory (Actin-binding proteins, GTPases and kinases) proteins are juxtaposed with transmembrane proteins. This protein scaffolding facilitates the assembly of highly ordered structures, such as junctional complexes or signaling patches that regulate epithelial cell polarity, proliferation and differentiation (Ref.2).
Tight Junctions are located at the uppermost portion of the lateral plasma membrane, where the integral membrane proteins like Claudins appear to be involved in the homophilic and/or heterophilic interactions implicated in firm adhesions. Claudins have four hydrophobic transmembrane domains and two extracellular loops (the first loop is larger than the second). The extracellular loops, whose sequences are distinct in different Claudins, contribute to the formation not only of Tight Junction strands but also of ion-selective channels. Among all Claudins, Claudin-1 is rather ubiquitous whereas Claudin-6 is developmentally restricted and not expressed in adult tissues. Claudin-5 is considered to be endothethial cell specific. In general Tight Junction strands are linear co-polymers of Occludin, various Claudins and JAMs that attract cytoplasmic proteins containing PDZ domains have high affinity for the C-terminal sequences of these proteins. Several membrane proteins that participate in Tight Junction-scaffolding bind to the C-terminal YV sequences of several Claudins through their PDZ domains (Ref.3 & 4). Those which directly interact with C-terminus of Claudins include ZO1, ZO2, ZO3, Afadin, MUPP1 (Multiple PDZ Domain Protein-1), PATJ (Pals1-Associated Tight Junction Protein), PILT (Protein Incorporated Later into Tight Junctions), Spectrin/Fodrin, transcription factor ZONAB, aPKC (Atypical Protein Kinase-C), Protein 4.1 and F-Actin. ZO1 is also directly regulated by GPCR (G-Protein Coupled Receptor)/GN-Alpha12 (Guanine Nucleotide-Binding Protein-Alpha-12); whereas GPCR/GN-Alpha/Ras-based signaling activates ZO1 associated Afadin to establish firm adhesions. PATJ interacts with PALS1 (Protein Associated with Lin7-1 (Mouse Homolog)), Crb1 and Crb3 to form a tripartite Tight Junction complex involved in epithelial cell polarity (Ref.2).
It is most likely that aPKC binds to PALS1-PATJ-Crb, and phosphorylates Crb. aPKC also associates with the PAR-3 (Partitioning Defective-3)-PAR-6 polarity complex that is recruited to Tight Junction. PAR-3 and PAR-6 interacts with Crb and PALS1 complex and results in Tight Junction assembly and apicobasal polarity. The interaction between PAR-6 and PALS1 is regulated by CDC42 (Cell Division Cycle-42). PAR-6 interaction with GTP-bound CDC42, a key modulator of the Actin cytoskeleton, results in the activation of aPKC at sites of cell-cell junctions. Interestingly, the small GTPase CDC42 regulates different steps of polarized membrane trafficking, such as basolateral to apical transcytosis, apical endocytosis and biosynthetic transport (Ref.5). It is possible that the binding of CDC42 to PAR-6 links the Tight Junction PAR-3/PAR-6/aPKC/Crb/PALS1/PATJ/TIAM1 (T-Cell Lymphoma Invasion and Metastasis-1)/MARK2 (MAP/Microtubule Affinity-Regulating Kinase-2) complexes to a signaling pathway regulating F-Actin/Myosin/Tubulin cytoskeleton, polarized membrane transport and Tight Junction assembly. Furthermore, aPKC, PAR-6 and mLGL (Mammalian Lethal Giant Larvae) form another multi-protein complex in which mLGL is phosphorylated by aPKC. mLGL phosphorylation is required for its localization along the lateral membrane and regulates the ability of mLGL to interact with Stx4 (Syntaxin-4) and thus to direct protein trafficking. However, aPKC function is inhibited by PP2A (Protein Phosphatase-2A) (Ref.6).
Unlike Claudin, Occludin is a transmembrane phosphoprotein expressed in the Tight Junctions of both epithelial and endothelial cells. Occludin is likely to be involved in establishing the seal at the sites of junctional strands. Occludins directly interacts with ZO1, ZO2, ZONAB, VAP33 (VAMP (Vesicle-Associated Membrane Protein)-Associated Protein-A-33kDa), PALS2, ZAK (Sterile Alpha Motif and Leucine Zipper Containing Kinase-AZK), Cttn (Cortactin), Spectrin/Fodrin, Protein 4.1, Ctnn-Beta (Catenin-Beta), Ctnn-Alpha, Alpha-Actn (Alpha-Actinin), Afadin, PILT, Cgn (Cingulin), 7H6 Antigen and Sympk (Symplekin). The cytoplasmic plaque proteins like Cgn and Sympk participates in nuclear as well as cytoplasmic polyadenylation and activates CSTF (Cleavage Stimulation Factor 3' pre-RNA Subunit) and HSF1 (Heat Shock Transcription Factor-1) to regulate mRNA stability and localization, and cell adhesion/Apoptosis, respectively (Ref.7). Cttn/Ctnn-Beta/Alpha-Actn activates Actin-related proteins ARP2/3 to coordinate the initiation of new filaments. ZO2 recruits aPKC, Rab13, PKA (Protein Kinase-A) and other RabGTPases to facilitate vesicular trafficking and to recruit Claudin1 and ZO1 to the Tight Junction. Both PKA and aPKC control particular vesicle-mediated transport steps. Rab13 interacts directly with PKA and inhibits PKA-dependent phosphorylation of VASP (Vasodilator-Stimulated Phosphoprotein) that is essential for Actin-remodelling. Further cAMP/PKA/RabGTPases activity stimulate apically directed transcytosis and secretion in epithelial cells, budding of constitutive transport vesicles from the trans-golgi network to the cell surface. Rab proteins are therefore required to control PKA activities required for vesicle transport at different membrane compartments. Analogously, phosphorylation of SNAREs (SNAP Receptors), which includes v-SNARE (Vesicle-associated SNARE) and t-SNARE (Target-membrane SNARE) proteins by PKA is implicated in the regulation of vesicle release (Ref.8 & 9).
Further cytokines like TNF-Alpha (Tumor Necrosis Factor-Alpha) and TGF-Beta (Transforming Growth Factor-Beta) regulate Occludin levels near junction points. TNF-Alpha/TNFR (Tumor Necrosis Factor Receptor) activates the Itg (Integrin)/ILK (Integrin-Linked Kinase)/GSK3 (Glycogen Synthase Kinase)/p130Cas (Crk-Associated Substrate-P130)/JNK (c-Jun Kinase) signaling and perturb the stability of the Tight Junction barrier (Ref.10). To optimize the loss scaffold proteins like MAGI2 (Membrane Associated Guanylate Kinase Inverted-2) and MAGI3 bind to Ctnn-Beta and Vcl (Vinculin) at Occludin junctions to prevent PTEN (Phosphatase and Tensin Homolog) degradation, which then significantly decreases the cell proliferation activity of the Akt (v-Akt Murine Thymoma Viral Oncogene Homolog) through conversion of PIP3 (Phosphatidylinositol-3,4,5-Trisphosphate) to PIP2 (Phosphatidylinositol-4,5-Bisphosphate) and prevents junction disassembly. Increase of PTEN activity also suppresses the Itg/ILK/GSK3/p130Cas/JNK signaling by decreasing Akt-induced GSK3 activation and this alters the level of Occludins near the Tight Junctions. Similarly, TGF-Beta/TGF-BetaR (Transforming Growth Factor-Beta Receptor) binds firmly to Occludins and regulates junction dynamics by promoting PAR-3 induced cell adhesion near Occludin and JAM junctions (Ref.11).
The components of the PAR-3/PAR-6/aPKC complex near JAMs regulate several signaling mechanisms that control epithelial polarization. PAR-3 interacts with the cytoplasmic domains of JAMs and hence mediates junctional recruitment of the complex. PAR-3 regulates Tight Junction assembly through PAR-6/aPKC-independent mechanism by regulating Rac1 activation via TIAM1 to formulate F-Actin/Myosin binding and cell adhesion. aPKC and CDC42 regulates vesicular trafficking, organization of the microtubule network and polarized membrane traffic (Ref.12). Apart from these other cell adhesion regulators like ZO1, ZONAB, Protein 4.1, Afadin, Spectrin/Fodrin, PILT, Cgn, CASK (Calcium/Calmodulin-Dependent Serine Protein Kinase (MAGUK Family)), MAGI1, Alpha-Actn, F-Actin and Myosin also form plaques near the cytoplasmic domains of JAMs to promote firm adhesions. Tight Junction formation (JAMs/Cgn complex) contributes to the down-regulation of RhoA activation and RhoA effector pathways like RhoA Signaling and Actin-Based Motility in high-density epithelial cells by inhibiting GEFH1 (Guanine Nucleotide Exchange Factor-H1) and this influences cell migration and cell cycle progression. By contrast, PAR-6 is also linked to the loss of the epithelial phenotype; TGF-Beta-induced epithelial-mesenchymal transition requires PAR-6 phosphorylation by TGF-BetaR. Phosphorylation triggers an interaction with the ubiquitin ligase SMURF1 (E3 Ubiquitin Ligase SMURF1), which has been proposed to target junction-associated RhoA for degradation and, hence, to induce disintegration of the junctional complex. Likewise PP2A induce disintegration of the junctional complex through direct inhibition of JAMs (Ref.2 & 11).
Tight Junctions also regulate epithelial proliferation by different molecular mechanisms, which generally suppress proliferation as well as cell density (and hence Tight Junction assembly) increases. Several proteins that localize to Tight Junctions as well as the nucleus lead to regulate gene expression. One of them is ZO1 that regulates proliferation and interacts with the Y-box transcription factor ZONAB, a protein that is required for normal proliferation rates. ZONAB regulates G1/S phase progression by two different mechanisms. First, it interacts with the G1/S phase regulator CDK4 (Cyclin-Dependent Kinase-4); hence, cytoplasmic sequestration of ZONAB by ZO1 results in reduced nuclear CDK4. Secondly, ZONAB functions in the transcriptional regulation of cell cycle regulators. Thus, the cytoplasmic sequestration of ZONAB and CDK4 results in co-regulation of two different mechanisms that affect G1/S phase transition. Another such protein is ZO2, which interacts with ZO1, enters the nucleus in proliferating cells and then binds with the hnRNP (mRNA-binding Protein), SAFB (Scaffold Attachment Factor-B) to inhibit the transcription factors, AP-1 (Activator Protein-1) and CEBP (CCAAT Enhancer Binding Protein) leading to the deregulation of epithelial cell proliferation and differentiation (Ref.1 & 13). Tight Junctions basically have two important functions in the establishment of epithelial barriers; first, they regulate formation of the barriers by modulating cell proliferation, differentiation and polarization, and second, they control barrier function by restricting paracellular diffusion. The above mechanisms shed insights on the regulation of paracellular permeability and may pave way for new therapeutic strategies in drug delivery across epithelial barriers (Ref.14 & 15).
References
- 1
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function.
- 2
- Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation.
- 3
- Van Itallie CM, Anderson JM. Claudins and Epithelial Paracellular Transport.
- 4
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies.
- 5
- Turksen K, Troy TC. Barriers built on claudins.
- 6
- Kohler K, Zahraoui A. Tight junction: a co-ordinator of cell signalling and membrane trafficking.
- 7
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1.
- 8
- Zahraoui A. Properties of Rab13 interaction with protein kinase A.
- 9
- Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface.
- 10
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier.
 关于我们
关于我们
