Ephrin-EphR_Signaling
发布时间:2019-12-10 10:22 来源:SABiosciences
- 通路
- 概述
Review
In numerous processes that are vital for the development and maintenance of organism function, cells must communicate crucial information to respond appropriately to the changing environment. As such, RTKs (Receptor Tyrosine Kinases) are transmembrane proteins, which, on receiving an external stimulus, respond by transmitting a signal to the inside of the cell. Of all the RTKs that are found in the human genome, the Eph Receptor family and their ligands the Ephrins, constitutes the largest family.
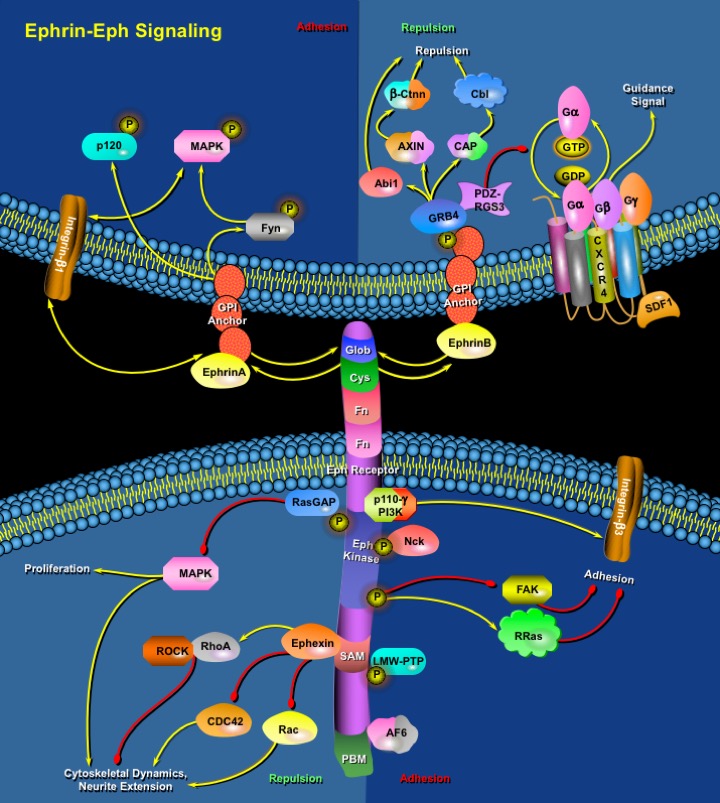
The Eph family of RTKs which has 14 members and their ligands, the Ephrins, are prominently involved in several developmental processes such as boundary formation, cell migration, axon guidance, synapse formation and angiogenesis. In vertebrates, Eph Receptors are divided into two groups, the EphA class (which contains eight members EphA1-EphA8), with EphA Receptors interacting preferentially with GPI (Glycosylphosphatidylinositol)-anchored Ephrin-As and the EphB class (which in mammals contains five members EphB1-EphB4, Eph6) with EphB Receptors binding to transmembrane anchored Ephrin-Bs. Characteristics of this family are the high promiscuity in interaction between Ephs and Ephrins and the capacity for bi-directional signaling, meaning that both Eph Receptors and Ephrins serve as receptors and as ligands (Ref.1). It is also a characteristic of the Eph Receptor family that their ligands must be membrane bound to be active. This requirement for membrane anchorage of the ligand makes cell-cell contact an obligatory event for activation of Eph Receptors, and consequently, the activated receptors are concentrated in the area of cell-cell contact. In accordance with their membrane-anchored nature, Ephrins are also involved in the process of reverse signaling. They interact with cytoplasmic signaling molecules and upon stimulation with appropriate receptors transmit signals inside the cell (Ref.2). Eph Receptors and their ligands are typically most highly expressed in neural and endothelial cells, and most descriptions of their function concern development of the nervous system and angiogenesis.
The Eph Receptor extracellular domain is composed of the ligand-binding Glob (Globular domain), a Cys (Cystein)-rich region and two FN (Fibronectin) Type-III repeats. The cytoplasmic part of Eph Receptors is divided into four functional units; the juxtamembrane domain that contains two conserved residues, a classical protein tyrosine kinase domain (Eph Kinase), a SAM (Sterile Alpha Motif) and PBM (PDZ-domain Binding Motif). Upon the formation of cell-cell contact, signaling through the Eph Receptors results in modulation of integrin activity and reorganization of the actin cytoskeleton. As a result, Ephs generate adhesive or repulsive signals, and in the neural system guide the movement of axonal growth cones, cell migration, and synapse formation (Ref.2). On ligand engagement each member of the receptor dimmer autophosphorylate several tyrosine residues that is located in the intracellular part of the partner receptor. Autophosphorylation of juxtamembrane tyrosine residues is required for full activation of the protein tyrosine kinase domain of the receptor (Ref.3). Once the receptor is activated, adaptor molecules associate with it to transmit signals into the cell. The Eph Receptors regulate actin dynamics through small GTPases including RhoA, R-Ras, RasGAP (Ras GTPase Activating Protein), ROCK (Rho-Associated Coiled-Coil-Containing Protein Kinase), and the Eph Receptor-interacting nucleotide exchange factor Ephexin, which can activate RhoA through Eph Kinase activation or directly. In neurons, Rho activation inhibits neurite outgrowth and promotes growth cone collapse and axon retraction (Ref.4). EphA Receptors can directly activate Rho GTPases through Ephexin. EphB Receptors interact with a different group of exchange factors for Rho family GTPases. EphB2 associate with the exchange factors Intersectin and Kalirin. Intersectin activates CDC42 and Kalirin, an exchange factor for Rac, co-clusters with activated EphB2 and localize activated Rac to sites of EphB-Ephrin interaction without changing the overall level of Rac activation. These interactions regulate the EphB Receptor-mediated morphogenesis and maturation of dendritic spines in cultured hippocampal and cortical neurons (Ref.5).
Other proteins involved in Eph-Ephrin signaling include LMW-PTP (Low Molecular Weight Protein Tyrosine Phosphatase), the SH2 and SH3 adaptor proteins Nck, GRB4 (Growth Factor Receptor Bound protein) and GRB10, SHEP1 (SH2 domain-containing Eph receptor-binding protein-1), FAK (Focal Adhesion Kinase), the p110Gamma isoform of PI3K (Phosphatidylinositol 3-Kinase), AF6 (Acute myeloid leukemia-1/chromosome6 Fusion protein) and p120, a 120 kD raft protein that is tyrosine phosphorylated in response to Ephrin-A5. Nck also links Eph receptors to the regulation of the actin cytoskeleton by cooperating with Rho proteins. Abl (Abelson), Src, and Fyn bind the juxtamembrane region of EphB2 through a phosphorylation-independent interaction with EphB2. Activated Eph Receptors suppress the MAPK (Mitogen Activated Protein Kinase)/ERK (Extracellular-Signal Regulated Kinase) pathway, which lead to growth-cone collapse and neurite retraction. Ephrin-Bs can also initiate reverse signaling through PDZ-RGS3-mediated associations, which leads to clustering and tyrosine phosphorylation of the cytoplasmic tail of Ephrin-B1, and a concomitant recruitment of GRB4 and its SH3-binding partners. The GTPase-activating protein PDZ-RGS3 catalyzes the hydrolysis of GTP to GDP in the G-Alpha subunit of heterotrimeric GPCR (G-protein Coupled chemokine Receptor), bind to the PDZ-binding motif of Ephrin-B molecules. It also inhibits SDF1 (Stromal Cell Derived Factor-1)-mediated cerebellar granule cell chemotaxis through the CXCR4 (Chemokine (C-X-C motif) Receptor-4) GPCR (Ref.6).
Interactions between Eph receptors and their ligands are critically important in many key developmental processes. Emerging evidence also supports a role for these molecules in postembryonic tissues, particularly in pathological processes, including tissue injury and tumor metastasis. Eph bi-directional signals are important for inhibiting cell intermingling between adjacent rhombomeres in Zebrafish, and similar mechanisms probably determine the segmental identity of the somites and the regional migration of neural crest cells. Eph Receptors and ephrins also define parasagittal stripes of Purkinje cells in the developing cerebellum (Ref.7). Eph RTK represents promising disease targets because they are differentially expressed in pathologic versus normal tissues. In addition to being present in tumor endothelial cells, EphA2 and Ephrin-A1 are up-regulated in the transformed cells of a wide variety of tumors including breast, prostate, colon, skin, and esophageal cancers. EphB proteins contribute to the organization of the vascular network by mediating neuro-arterial interactions. These findings have important implications for understanding and treating cancer because Eph proteins probably regulate angiogenic processes associated with tumor growth. EphB4 and Ephrin-B2 also play a role in erythropoiesis by influencing hematopoietic cell lineages that share a common ancestry with endothelial cells. Furthermore, Eph proteins play a role in platelet clustering and hence may be important for blood clotting at sites of vascular injury (Ref.8). Eph proteins mainly determine the initial sorting and positioning of cells in a wide spectrum of neural tissues.
References
- 1
- Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer.
- 2
- Augustin HG, Reiss Y. EphB receptors and ephrinB ligands: regulators of vascular assembly and homeostasis
- 3
- Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo.
- 4
- Yuan XB, Jin M, Xu X, Song YQ, Wu CP, Poo MM, Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance.
- 5
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin.
- 6
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus.
- 7
- Santiago A, Erickson CA Ephrin-B ligands play a dual role in the control of neural crest cell migration.
- 8
- Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred.
 关于我们
关于我们