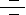CD40_Signaling
发布时间:2019-12-09 19:45 来源:SABiosciences
- 通路
- 概述
Review
CD40, a TNFR (Tumor Necrosis Factor Receptor) family member, conveys signals regulating diverse cellular responses, ranging from proliferation and differentiation to growth suppression and cell death. First identified and functionally characterized on B-Cells, CD40 is expressed on a plethora of different cell types, including B-Cells, macrophages, dendritic cells, endothelial cells, and fibroblasts, and this widespread expression accounts for the central role of CD40 in the regulation of immune response and host defense (Ref.1). Binding of CD40 with its counter receptor, CD154 (also termed CD40L [CD40 ligand] or GP39), acts on Antigen presenting cells and T-Cells in a bi-directional fashion, mediating both humoral and cellular immune responses (Ref.2).
Unique to particular types of cells, CD40 engagement sets in motion a pattern of gene expression. B-Cells depend on CD40 for survival, for expression of costimulatory molecules like B7 (to interact with T-Cells), germinal center formation, memory generation, Ig (Immunoglobulin) class switching and production of numerous cytokines and chemokines (IL-1, IL-6, IL-8, IL-10, IL-12 [Interleukins], TNF-Alpha [Tumor Necrosis Factor-Alpha], MIP1Alpha [Macrophage Inflammatory Protein-1Alpha] and cytotoxic radicals (Ref.3). Vascular endothelial cells after CD40 triggering produce cytokines such as IL-1 and IL-8, express COX2 (Cyclooxygenase-2), and display an increased density of cell adhesion molecules. CD40-CD40L interactions can influence T-Cell priming and T-Cell–mediated effector’s functions and activate macrophages, NK (Natural Killer) cells, and endothelia as well as participate in organ-specific autoimmune diseases, graft rejection, and even atherosclerosis. On the surface of dendritic cells, CD40-CD40L ligation regulates production of certain proinflammatory cytokines such as IL-8, MIP-1Alpha, TNF-Alpha and IL-12. Ligation of CD40 on monocytes is important in stimulating production of IL-1Alpha, IL-1Beta, TNF-Alpha, IL-6, and IL-8, as well as in the rescue of circulating monocytes from apoptotic death. Cell-to-cell contact via CD40-CD40L interactions is required for production of NO (Nitric Oxide) and IL-12 by macrophages. NK cells induce B-Cell maturation, immunoglobulin secretion, and isotype switching—the pathways normally regulated by CD40-CD40L interactions. CD40 activates p44/42 MAPK through the Ras pathway in neuronal cells. CD40-CD40L interactions may also regulate proliferation and activation of effector cells such as mast cells, eosinophils, smooth muscle cells, and, most recently, human cultured myoblasts. Evidence of CD40 expression on non-immune cell types suggests a broader role of CD40 in cellular biology (Ref. 4).
CD40-mediated signal transduction induces the transcription of a large number of genes implicated in host defense against pathogens. This is accomplished by the activation of multiple pathways including NF-KappaB (Nuclear Factor-KappaB), MAPK (Mitogen-Activated Protein Kinase) and STAT3 (Signal Transducers and Activators of Transcription-3) (Ref.2) that regulate gene expression through activation of Activating Proteins, c-Jun, ATF2 (Activating Transcription Factor-2) and Rel transcription factors (Ref.3). Receptor clustering of CD40L is mediated by an association of the ligand with p53, a translocation of ASM (Acid Sphingomyelinase) to the cell membrane, an activation of the ASM, and formation of ceramide. Ceramide appears to modify preexisting sphingolipid-rich membrane micro domains to fuse and form ceramide-enriched signaling platforms that serve to cluster CD40L (Ref.5). Several TRAF (TNF Receptor Associated Factor) proteins, including TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6, have been shown to interact with CD40 and to play critical roles in CD40-mediated pathways (Ref.6). TRAF2, TRAF3 and TRAF6 bind to CD40 directly and efficiently. TRAF1 does not directly bind CD40 but can be recruited to membrane micro domains through heterodimerization with TRAF2. Analogous to the recruitment of TRAF1, TRAF5 is also indirectly recruited to CD40 in a TRAF3-dependent manner (Ref.2). Act1 (NF-KappaB Activator-1) functions as an adapter, linking TRAF proteins to TAK1 (Transforming growth factor-Beta Activated Kinase-1) /IKK (I-KappaB kinases) to activate NF-KappaB/I-KappaB (Inhibitor of Kappa Light Chain Gene Enhancer in B-Cells), and MKK (Mitogen-Activated Protein Kinase Kinase) complex to activate JNK, p38 MAPK and ERK1/2. NIK (NF-KappaB-Inducing Kinase) also plays a leading role in activating IKK. Act1-dependent CD40-mediated NF-KappaB activation protects the cells from CD40L-induced apoptosis. On stimulation with CD40L or other inflammatory mediators, the I-KappaB proteins are phosphorylated by IKK and NF-KappaB is activated by over expression of Act1 through the TAK1 and IKK signaling cascade. Phosphorylated I-KappaB is then rapidly ubiquitinated and degraded. The liberated NF-KappaB translocates to the nucleus and activates transcription (Ref.7). A20, which is induced by TNF inhibit NF-kappaB activation as well as TNF-mediated apoptosis. TRAF3 can initiate signaling pathways that lead to the activation of p38 and JNK (c-Jun NH2-terminal kinase) but inhibits Act1-dependent CD40-mediated NF-KappaB activation and initiates CD40L-induced apoptosis. TRAF2 is required for activation of SAPK (Stress-Activated Protein Kinase) pathways and also plays a role in CD40-mediated surface molecule upregulation, IgM secretion in B-Cells and up-regulation of ICAM1 (Intercellular Adhesion Molecule-1) gene (Ref.6).
CD40 ligation by CD40L stimulates MCP1 (Monocyte Chemoattractant Protein-1) and IL-8 production in primary cultures of human proximal tubule cells and that this proceed primarily via recruitment of TRAF6 and activation of the ERK1/2 (Extracellular Signal Regulated Kinases), the SAPK/JNK and p38 MAPK pathways. Activation of SAPK/JNK and p38 is mediated via TRAF6 whereas ERK1/2 activity is potentially mediated via other TRAF members. However, stimulation of all three MAPK pathways is required for MCP1 and IL-8 production (Ref.8). Other pathways activated by CD40 stimulation include the JAK3 (Janus family of kinases)-STAT3 and PI3K (Phosphatidyl Inositol 3-Kinase)-Akt, which may contribute to the antiapoptotic properties conferred by CD40L in B-Cells (Ref.9). CD40 directly binds to JAK3 and mediates STAT3 activation followed by up-regulation of ICAM1, CD23, and LT-Alpha (Lymphotoxin Alpha).
In human beings, mutations in CD40L lead to an impaired ability of B-Cells to undergo Ig class switching, abnormal antibody levels, and an inability to respond to certain bacterial infections. Aberrant expression of CD40 has been associated with autoimmune inflammatory diseases such as multiple sclerosis and rheumatoid arthritis. The receptor is also expressed on epithelial carcinomas and has been shown to promote spontaneous and chemotherapeutic drug-induced apoptosis in epithelial cancer cell lines CD40 ligation leads to the control of Trypanosoma cruzi infection through the induction of NO (Nitric Oxide). CD40-CD40L interactions are required for a broad spectrum of anti-infective host immune responses induced following infection with bacteria, parasites or viruses. A CD40-CD40L-mediated protective effect is also observed with other pathogens such as Cryptosporidium parvum, Pneumocystis carinii and Leishmania infection (Ref.10) Humans that have a mutation in CD40L develop a severe form of immunodeficiency, HIGM1 (Hyper IgM syndrome), that is characterized by elevated levels of IgM in the majority of patients and low levels of IgA, IgG, and IgE, the absence of germinal centers, and the inability to mount thymus derived humoral response (Ref. 4). CD40L stimulating properties have already been used in the treatment of tumors, tumor regression being linked to restoration of MHC1 (Major Histocompatibility Complex Class-1) expression by tumor cells, IL-12 overproduction, or potentiation of host Antigen Presenting Cell functions. Moreover, CD40 engagement restores at least in vitro, production of IL-12 by cells from HIV (Human Immunodeficiency Virus)-infected patients and it stimulates macrophages to produce HIV1-suppressive chemokines. Activation of the immune system through CD40 ligation may provide a potent strategy for immunotherapy of parasitic diseases (Ref.10).
References
- 1
- Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily.
- 2
- Pype S, Declercq W, Ibrahimi A, Michiels C, Van Rietschoten JG, Dewulf N, de Boer M, Vandenabeele P, Huylebroeck D, Remacle JE. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated fact
- 3
- Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes.
- 4
- Grewal IS, Flavell RA. CD40 and CD40L in cell-mediated immunity.
- 5
- Grassme H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to form a functional contact with CD40.
- 6
- Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1).
- 7
- Qian Y, Zhao Z, Jiang Z, Li X. Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells.
- 8
- Li H, Nord EP. CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells.
- 9
- Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily.
- 10
- Chaussabel D, Jacobs F, de Jonge J, de Veerman M, Carlier Y, Thielemans K, Goldman M, Vray B. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation.
 关于我们
关于我们