AHR_Pathway
发布时间:2019-12-09 18:09 来源:SABiosciences
- 通路
- 概述
Review
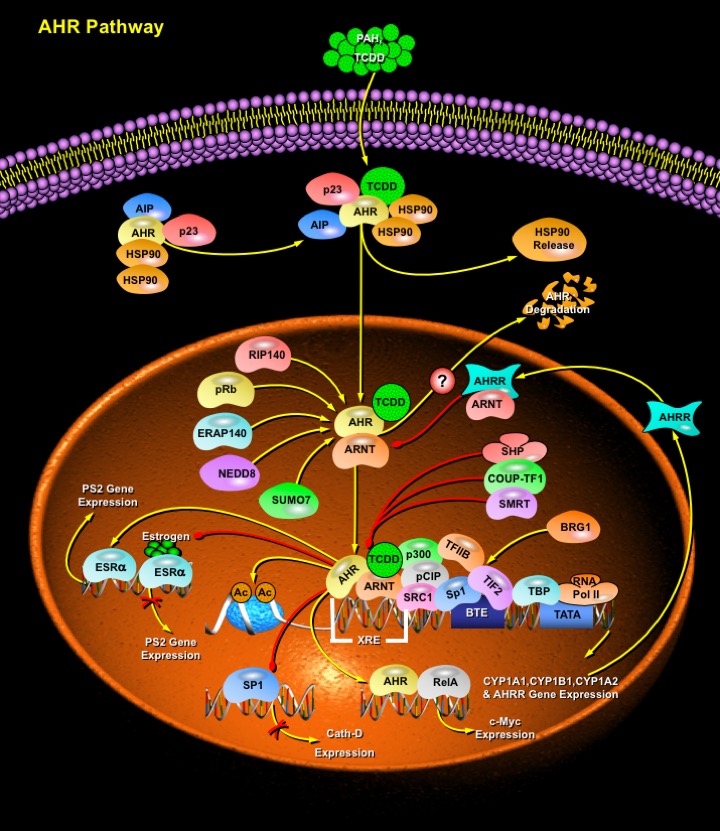
AHR (Aryl Hydrocarbon Receptor) is a member of the bHLH (basic Helix–Loop–Helix)- PAS (Per-ARNT-Sim) family of transcriptional regulators that control a variety of developmental and physiological events, including Neurogenesis, Tracheal and Salivary duct formation, Toxin metabolism, Circadian rhythms, response to Hypoxia and Hormone Receptor function. The unique feature of all bHLH-PAS proteins is the PAS domain, named after the first three proteins identified with this motif, the Drosophila Per, Human ARNT and Drosophila Sim. The PAS domain consists of 260–310 amino acids and incorporates two well-conserved hydrophobic repeats, termed PAS-A and PAS-B, separated by a poorly conserved spacer. Overall, the PAS domain is not well conserved and can mediate a number of diverse biochemical functions. AHR, also known as the Dioxin receptor, is recognized as the culprit for most toxic responses observed after exposure to PAH (Polycyclic Aromatic Hydrocarbons), Dioxins (e.g. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin)), and Polychlorinated Biphenyls. Ligands for AHR are diverse which include dietary compounds, natural and synthetic flavonoids, natural products, and pharmaceuticals. AHR can affect cellular signaling through interactions with various Regulatory and Signaling proteins, including PAS heterodimerization partners ARNT (Aryl Hydrocarbon Receptor Nuclear Translocator), Chaperone and Immunophilin-like proteins (e.g. HSP90 (Heat Shock Protein-90), AIP (Aryl Hydrocarbon Receptor-Interacting Protein), p23)), Protein Kinases and Phosphatases (e.g. Tyrosine Kinases, CK2 (Casein Kinase-2), PKC (Protein Kinase-C)), and Coactivators (e.g. SRC1(Steroid Receptor Coactivator-1), RIP140, CBP (CREB-Binding Protein)/p300). In addition, AHR is also known to interact with signaling pathways that are mediated by ESR (Estrogen Receptor) and other Hormone receptors, Hypoxia, NF-KappaB (Nuclear Factor-Kappa-B) and Rb (Retinoblastoma) protein (Ref.1 & 2).
Normally, AHR exists in a dormant state within the cytoplasm in association with a complex of HSP90, XAP2 (X-Associated Protein- 2), also known as ARA9 and AIP, and HSP90 Co-chaperone p23. Upon ligand binding, AHR in the complex is activated by a conformation change that exposes a NLS (Nuclear Localization Signal). HSP90 is released from the complex and the receptor translocates to the nucleus, where it forms a heterodimer with ARNT. The heterodimer binds to the XRE (Xenobiotic Response Element) and alters expression of genes controlled by enhancer XREs. XREs, with the conserved core sequences "GCGTG", are found in the promoter regions of several genes involved in the metabolism of xenobiotics, including CYP1A1 (Cytochrome P450 Family-1 Subfamily-A Polypeptide-1), CYP1A2 (Cytochrome P450 Family-1 Subfamily-A Polypeptide-2), CYP1B1 (Cytochrome P450 Family-1 Subfamily-B Polypeptide-1) and NAD(P)H-Quinone Oxidoreductase. Expression of CYP1A1 gene is regulated in a Substrate-inducible manner through at least two kinds of regulatory DNA elements in addition to the TATA sequence, XRE and BTE (basic transcription element), a GC box sequence. The transacting factor on the XRE is AHR/ARNT Heterodimer, while SP1 (Transcription Factor SP1) acts as a regulatory factor on the BTE. Binding of SP1 to the BTE facilitated the binding of the AHR/ARNT heterodimer to the XRE by physical interaction and vice versa, cooperatively enhancing expression of the CYP1A1 gene. The AHR/ARNT heterodimer bound to the XRE sequence and, in turn recruited CBP or p300, a HAT (Histone Acetyltransferase Complex) coactivator, to the C-terminal activation domain of ARNT (Ref.3 & 4). Several other nuclear receptor coactivators also interact with the AHR, including ERAP140, RIP140, BRG1 (Brahma-Related Gene-1), Rb, PML (Acute Promyelocytic Leukemia Inducer), NEDD8 (Neural precursor cell Expressed, Developmentally Down-regulated-8), SUMO1 (Small Ubiquitin Related Modifier-1) and the three members of the p160 family of coactivators: NCOA1 (Nuclear Receptor Coactivator-1)/SRC1, NCOA2 (Nuclear Receptor Coactivator-2)/GRIP1/TIF2, and NCOA3 (Nuclear Receptor Coactivator-3)/AIB1 /pCIP/ ACTR. Ordered and cyclical association of AHR and coactivators led to Histone acetylation, Pol II (RNA Polymerase-II) recruitment and gene transcription. In other cases, activation of the AHR results in transcriptional inhibition of genes such as those encoding the Immunoglobulin heavy-chain and Estrogen-inducible p27, Cathepsin D, and pS2 (Gastrointestinal Trefoil Protein-pS2) (Ref.5, 6 & 7). A novel PAS protein called AHRR (AHR Repressor) inhibits AHR signal transduction by competing with AHR for ARNT and also by binding to XRE. The AHRR is induced by AHR, thus forming a negative feedback loop for the regulation of AHR. PKC and Tyrosine Kinase are also involved in AHR signal transduction, as inhibitors of these kinases block the induction of target genes. Other negative regulators of AHR include SMRT (Silencing Mediator for Retinoic acid and Thyroid hormone receptor) COUP-TFI (Chicken Ovalbumin Upstream Promoter-Transcription Factor-1) and SHP (Small Heterodimer Partner). SMRT associates with the AHR and suppress CYP1A1 transactivation, whereas SHP inhibited AHR/ARNT-DNA binding. AHR has been shown to directly interact with the orphan receptor COUPTF1, which may regulate AHR by competition for DNA binding at DREs. Recent reports have suggested that AHR is rapidly downregulated following ligand binding by degradation. Xenobiotic-activated AHR is degraded by the Ubiquitin/Proteasome system after being exported from the nucleus to the cytoplasm. As the degradation of AHR is important for the regulation of AHR activity, determination of the physiological location of AHR degradation remains important for future research (Ref.8 & 9).
AHR also interacts with other signaling pathways such as those mediated by Estrogen Receptor and other Hormone receptors, Hypoxia, NF-KappaB and Rb. Perhaps the most studied evidence of cross talk with the AHR pathway concerns Steroid hormone receptors. Evidence of AHR interactions with ESR, AR (Androgen Receptor), and Thyroid Hormone Receptor pathways has been suggested. AHR activation lead to decrease in both ESR number and ESR responsiveness, as well as increases in ESR metabolism. Agonist-activated AHR–ARNT complexes associate directly with ESR-Alpha and ESR-Beta in the absence of Estrogen resulting in transcriptional activation of ERE-dependent genes. This ERE-dependent estrogenic effect of liganded AHR requires direct interaction of the nuclear AHR–ARNT complex with unliganded ESR and the cofactor p300/CBP. By contrast, in the presence of Estrogen, liganded AHR exhibits anti-Estrogenic effects by suppressing Estrogen-bound ESR-mediated DNA binding. AHR also interacts with NF-KappaB signaling pathways. Direct interactions between AHR and RelA (a NF-KappaB subunit) induce transactivation of c-Myc protein (Ref.2, 10 & 11). Functional cross talk between AHR and NF-KappaB occurred through interactions with common coactivators SRC1 and p300/CBP. AHR and NF-KappaB RelA form an inactive complex, thereby causing mutual repression. The association between the AHR and RelA provides a physical basis for the functional antagonism. Recently, it has been found that modulation of the PKC pathway also affects AHR signaling. However, neither direct interactions between any PKC pathway proteins nor phosphorylation of any AHR complex proteins by PKC have been reported. AHR may also be involved in cell-cycle regulation through growth factor signaling, cell-cycle arrest and apoptosis. Research on interactions between AHR and various cell signaling cascades has progressed slowly because mechanisms of AHR transactivation are still being uncovered and because of the complexity of interdependent protein–protein interactions. Promoter region analyses of known AHR-responsive genes help to clarify the role of AHR during embryonic development and cell homeostasis. Comparisons of human AHR signaling events with other species will provide insights into species-specific AHR mechanisms and may provide further evidence of an evolutionarily conserved, endogenous role for AHR. Finally, it remains to be determined whether nontranscriptional events mediated by the AHR play an important role in the biology and toxicology of this interesting receptor (Ref.1, 12 & 13).
References
- 1
- Bock KW, Kohle C. Ah receptor: Dioxin-mediated toxic responses as hints to deregulated physiologic functions.
- 2
- Hestermann EV, Brown M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor.
- 3
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes.
- 4
- Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 bindin
- 5
- Tojo M, Matsuzaki K, Minami T, Honda Y, Yasuda H, Chiba T, Saya H, Fujii-Kuriyama Y, Nakao M. The aryl hydrocarbon receptor nuclear transporter is modulated by the SUMO-1 conjugation system.
- 6
- Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, Nelson WE, Probst MR, Rosenfeld MG, Hankinson O. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon recep
- 7
- Wang F, Samudio I, Safe S. Transcriptional activation of cathepsin D gene expression by 17beta-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition.
- 8
- Bernshausen T, Jux B, Esser C, Abel J, Fritsche E. Tissue distribution and function of the Aryl hydrocarbon receptor repressor (AhRR) in C57BL/6 and Aryl hydrocarbon receptor deficient mice.
- 9
- Klinge CM, Jernigan SC, Risinger KE, Lee JE, Tyulmenkov VV, Falkner KC, Prough RA. Short heterodimer partner (SHP) orphan nuclear receptor inhibits the transcriptional activity of aryl hydrocarbon receptor (AHR)/AHR nuclear translocator (ARNT).
- 10
- Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells.
 关于我们
关于我们
